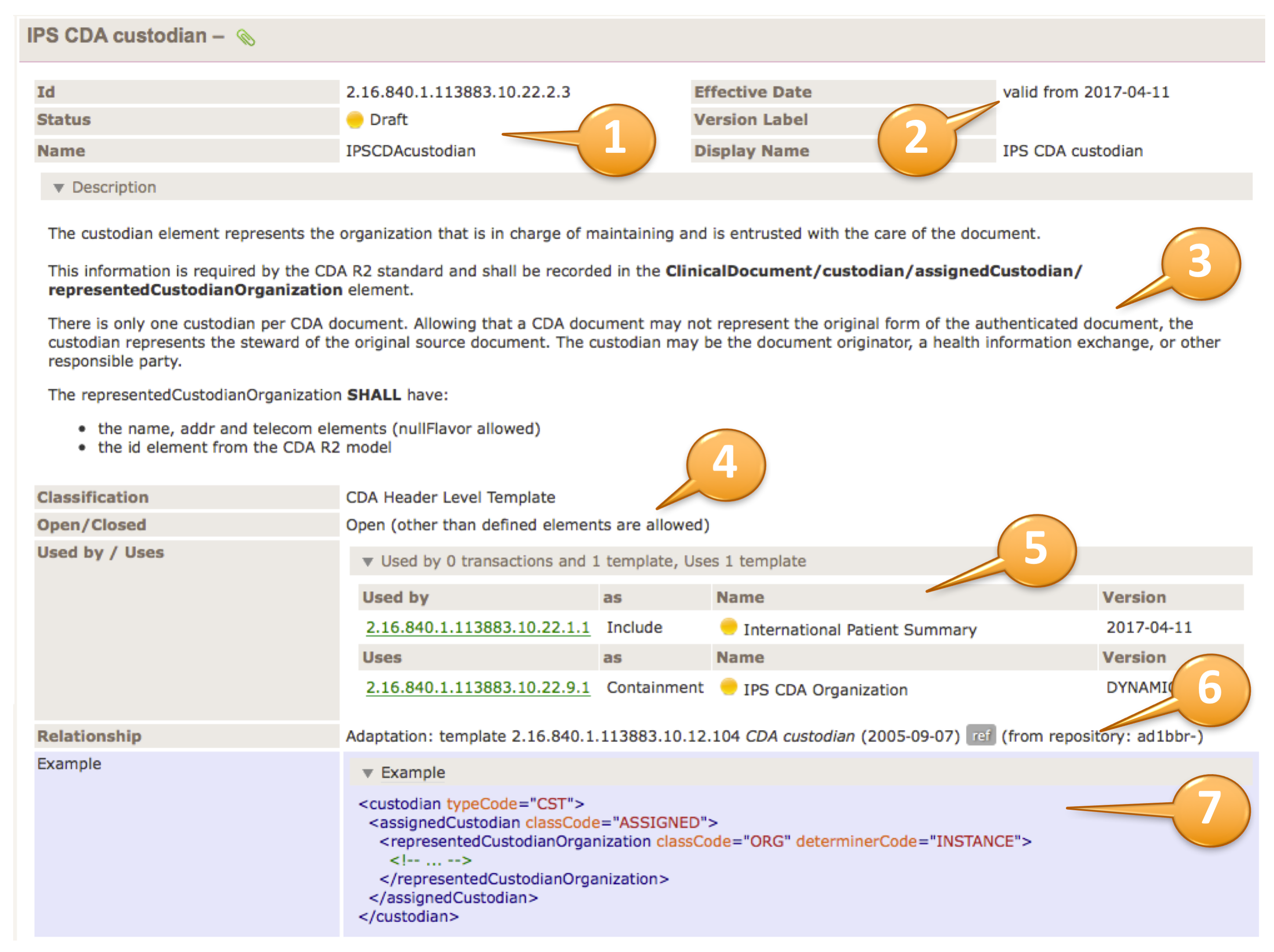
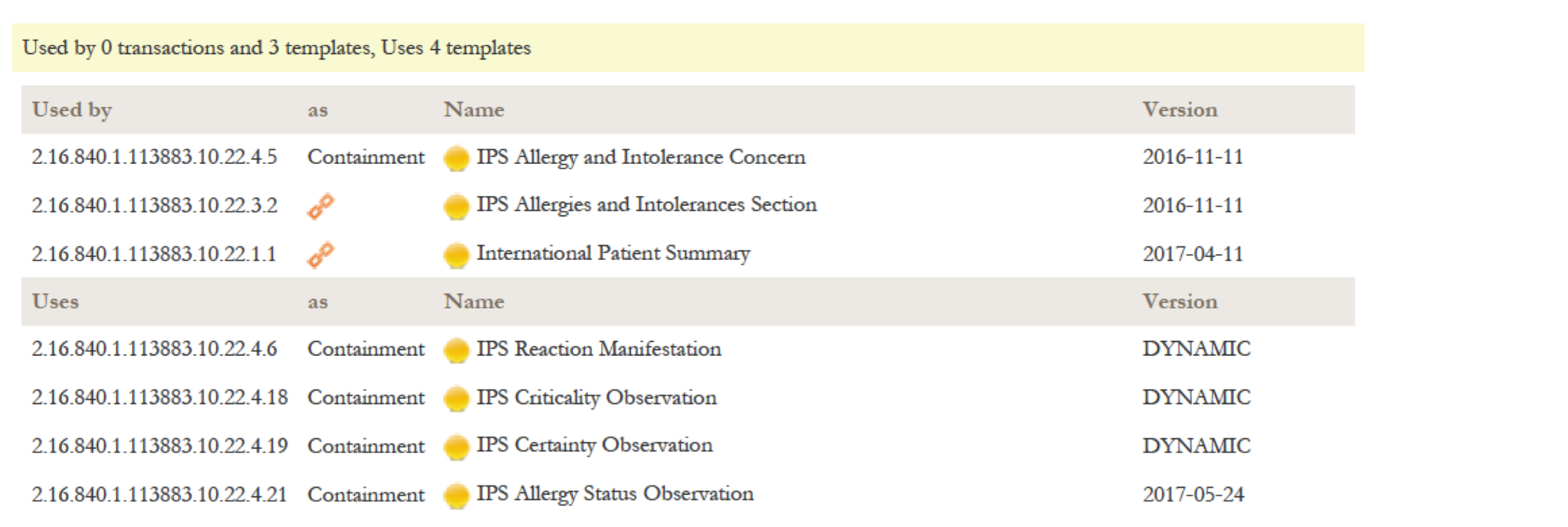
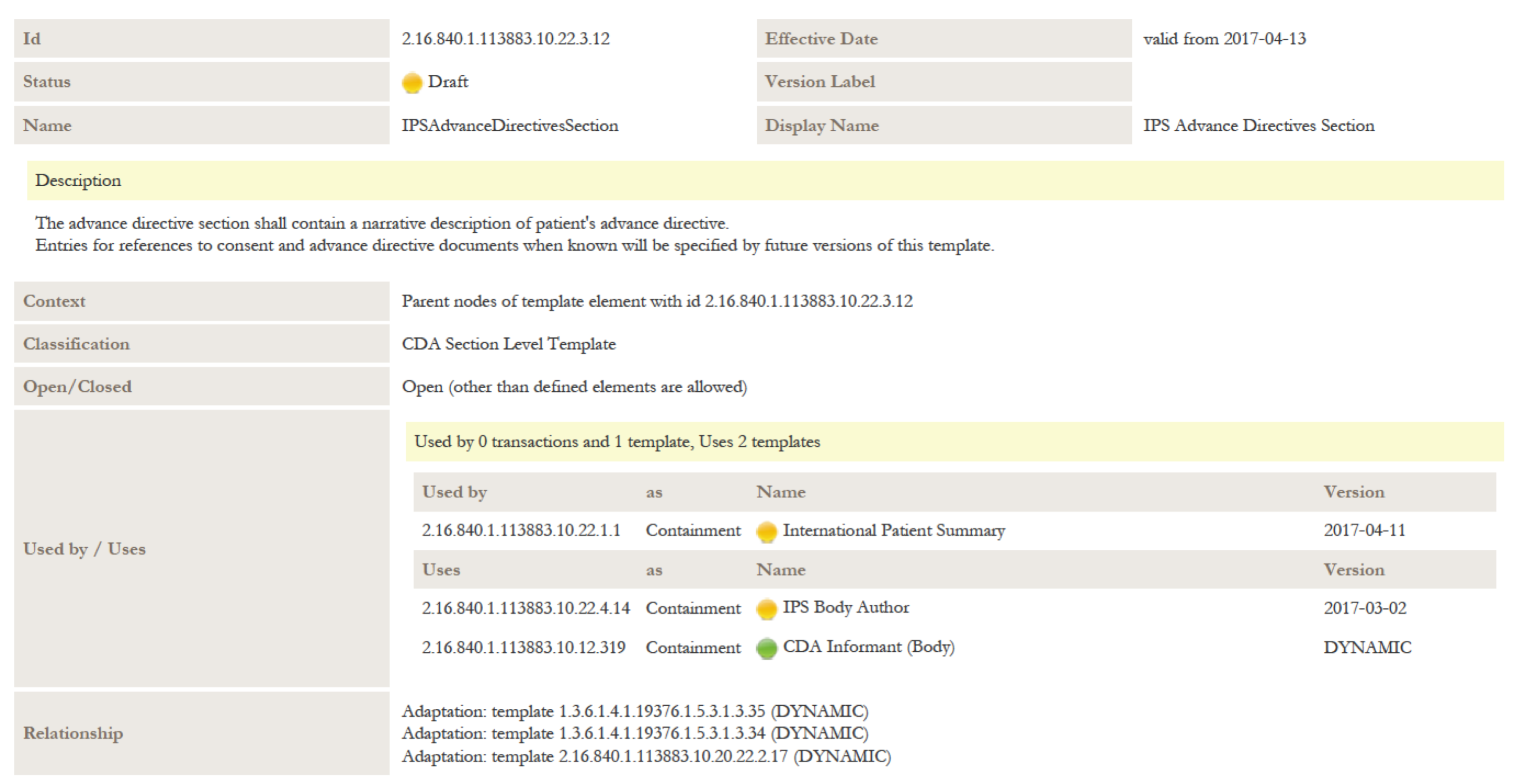
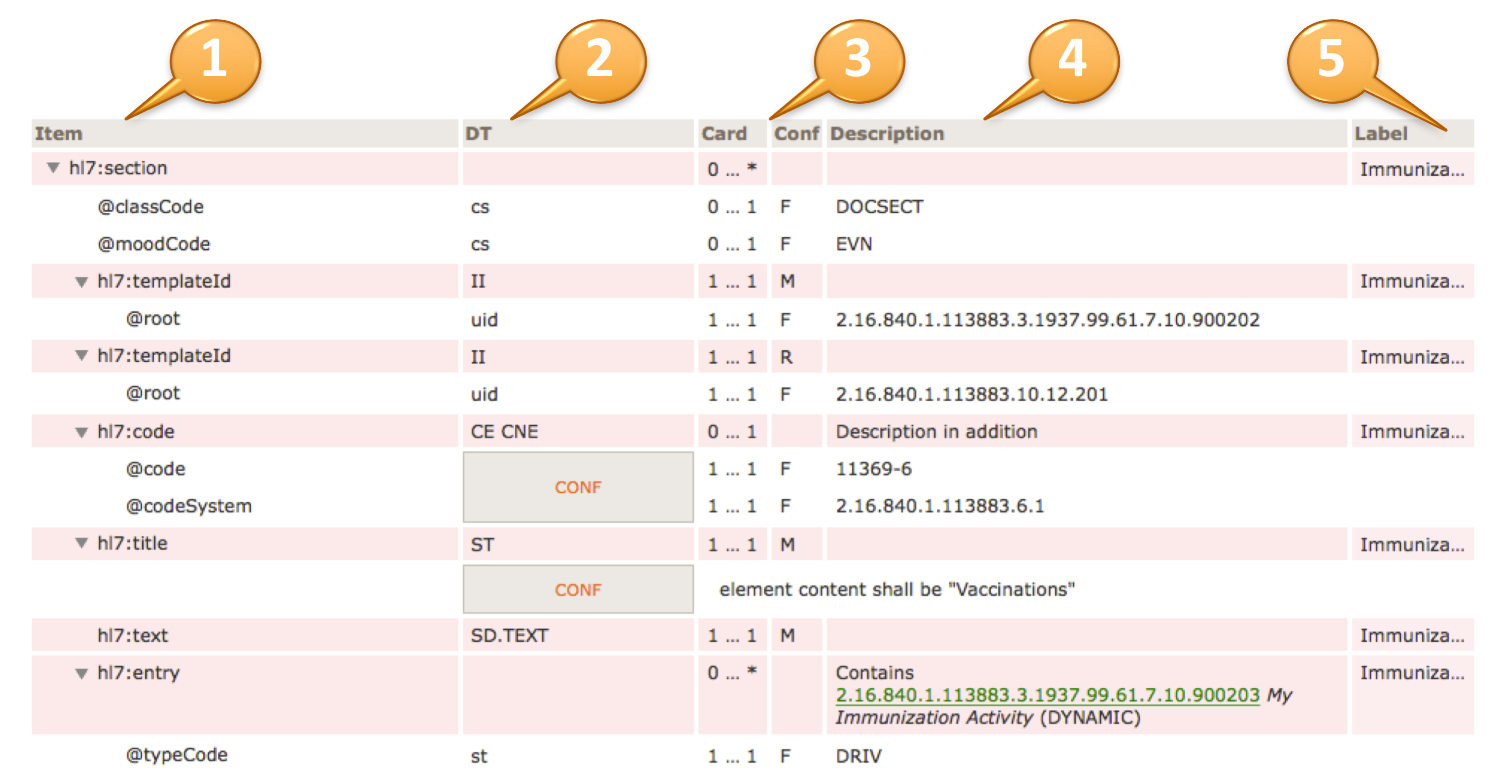
Important Notes
HL7 licenses its standards and select IP free of charge. If you did not acquire a free license from HL7 for this document, you are not authorized to access or make any use of it. To obtain a free license, please visit http://www.HL7.org/implement/standards/index.cfm.
If you are the individual that obtained the license for this HL7 Standard, specification or other freely licensed work (in each and every instance "Specified Material"), the following describes the permitted uses of the Material.
A. HL7 INDIVIDUAL, STUDENT AND HEALTH PROFESSIONAL MEMBERS, who register and agree to the terms of HL7’s license, are authorized, without additional charge, to read, and to use Specified Material to develop and sell products and services that implement, but do not directly incorporate, the Specified Material in whole or in part without paying license fees to HL7.
INDIVIDUAL, STUDENT AND HEALTH PROFESSIONAL MEMBERS wishing to incorporate additional items of Special Material in whole or part, into products and services, or to enjoy additional authorizations granted to HL7 ORGANIZATIONAL MEMBERS as noted below, must become ORGANIZATIONAL MEMBERS of HL7.
B. HL7 ORGANIZATION MEMBERS, who register and agree to the terms of HL7's License, are authorized, without additional charge, on a perpetual (except as provided for in the full license terms governing the Material), non-exclusive and worldwide basis, the right to (a) download, copy (for internal purposes only) and share this Material with your employees and consultants for study purposes, and (b) utilize the Material for the purpose of developing, making, having made, using, marketing, importing, offering to sell or license, and selling or licensing, and to otherwise distribute, Compliant Products, in all cases subject to the conditions set forth in this Agreement and any relevant patent and other intellectual property rights of third parties (which may include members of HL7). No other license, sublicense, or other rights of any kind are granted under this Agreement.
C. NON-MEMBERS, who register and agree to the terms of HL7’s IP policy for Specified Material, are authorized, without additional charge, to read and use the Specified Material for evaluating whether to implement, or in implementing, the Specified Material, and to use Specified Material to develop and sell products and services that implement, but do not directly incorporate, the Specified Material in whole or in part.
NON-MEMBERS wishing to incorporate additional items of Specified Material in whole or part, into products and services, or to enjoy the additional authorizations granted to HL7 ORGANIZATIONAL MEMBERS, as noted above, must become ORGANIZATIONAL MEMBERS of HL7.
Please see http://www.HL7.org/legal/ippolicy.cfm for the full license terms governing the Material.
Ownership. Licensee agrees and acknowledges that HL7 owns all right, title, and interest, in and to the Materials. Licensee shall take no action contrary to, or inconsistent with, the foregoing.
Licensee agrees and acknowledges that HL7 may not own all right, title, and interest, in and to the Materials and that the Materials may contain and/or reference intellectual property owned by third parties (“Third Party IP”). Acceptance of these License Terms does not grant Licensee any rights with respect to Third Party IP. Licensee alone is responsible for identifying and obtaining any necessary licenses or authorizations to utilize Third Party IP in connection with the Materials or otherwise. Any actions, claims or suits brought by a third party resulting from a breach of any Third Party IP right by the Licensee remains the Licensee’s liability.
Following is a non-exhaustive list of third-party terminologies that may require a separate license:
| Terminology | Owner/Contact |
|---|
| Current Procedures Terminology (CPT) code set | American Medical Association
https://www.ama-assn.org/practice-management/cpt-licensing |
| SNOMED CT© | SNOMED CT® International http://www.snomed.org/snomed-ct/get-snomed-ct or info@ihtsdo.org |
| Logical Observation Identifiers Names & Codes (LOINC©) | Regenstrief Institute, Inc. |
| International Classification of Diseases (ICD) codes | World Health Organization (WHO) |
| NUCC Health Care Provider Taxonomy code set | American Medical Association. Please see www.nucc.org. AMA licensing contact: 312-464-5022 (AMA IP services) |
Obtaining a CPT Sublicense from HL7
Contact hq@hl7.org about how to obtain a sublicense from HL7 for non-production use of CPT for (i) the development and publication of value sets, profiles, and other artifacts as part of the HL7 Implementation Guides, (ii) as part of defined VSAC value sets, and (iii) to support HL7's terminology services within the Territory.
Flow Down Clauses for CPT Sublicense from HL7
CPT content is copyrighted by the American Medical Association and CPT is a registered trademark of the AMA.
HL7, as a party to a license agreement with the AMA, is authorized to grant user a limited, non-exclusive, non-transferable, non-sublicensable license for user to use CPT content for (i) the development and publication of value sets, profiles, and other artifacts as part of the HL7 Implementation Guides, (ii) as part of defined VSAC value sets, and (iii) to support HL7's terminology services within the Territory, each of which shall be considered a non-production use. The sublicense granted hereunder shall automatically terminate upon termination of the agreement between HL7 and AMA, unless prior written consent of AMA is obtained.
The provision of updated CPT content is dependent on a continuing contractual relationship between HL7 and the AMA.
User acknowledge a separate license agreement shall be required, and shall govern any proposed use, including any distribution of CPT content for any other purposes not expressly permitted under this Agreement, and the terms of such agreement will govern such use (e.g., a separate license agreement shall govern production use and commercial purposes). AMA reserves the right to accept or reject licenses based on AMA's evaluation of the proposed use of the CPT content.
User acknowledge that User's development and commercialization of CPT-informed works developed with reference to Licensed Products may only be implemented in the Territory.
User is prohibited from making CPT content publicly available, creating derivative works (including translating), transferring, selling, leasing, licensing, or otherwise making available to any unauthorized party the CPT content, or a copy or portion of CPT content to any unauthorized party, including a subsidiary, affiliate, or other legal entity, however designated, for any purpose whatsoever except as expressly permitted under a separate agreement.
User expressly acknowledges and agrees to the extent permitted by applicable law, use of CPT content is at User's sole risk and CPT content is provided "as is" without warranty of any kind. The AMA does not directly or indirectly practice medicine or dispense medical services. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT, and the AMA is not recommending their use. CPT content herein does not replace the AMA's Current Procedural Terminology book or other appropriate coding authority. The coding information contained in CPT content should be used only as a guide.
U.S. Government End Users. CPT is commercial technical data, which was developed exclusively at private expense by the American Medical Association (AMA), 330 North Wabash Avenue, Chicago, Illinois 60611. This agreement does not grant the Federal Government a direct license to use CPT based on FAR 52.227- 14 (Data Rights - General) and DFARS 252.227-7015 (Technical Data - Commercial Items).
User expressly consents to the release of its name to the AMA.
| Co-Chair, Primary Editor |
Melva Peters
Jenaker Consulting
melva@jenakerconsulting.com
|
| Co-Chair, Primary Editor |
John Hatem
jnhatem@hotmail.com
|
| Co-Chair |
Scott Robertson PharmD
scott.m.robertson@kp.org
|
| Co-Chair |
Jean Duteau
jean@duteaudesign.com
|
| Contributor |
Dr Kai U. Heitmann
Heitmann Consulting and Services, ART-DECOR Open Tools GmbH, HL7 Germany
info@kheitmann.de |
| Contributor |
Giorgio Cangioli, PhD
Consultant, HL7 Italy
giorgio.cangioli@gmail.com
|
| Contributor |
Tom de Jong
VZVZ, HL7 The Netherlands
tom@nova-pro.nl |
| Contributor |
Dr Christof Geßner
Gematik GmbH, HL7 Germany
christof.gessner@gematik.de |
Introduction
This Implementation Guide provides CDA R2 templates for Medication Order and Medication Statement, Medication Dispense and Medication Administration that can be used by HL7 standards developers and external projects to develop models for pharmacy related content. The implementation guide is intended to provide consistency of pharmacy related models across all uses regardless of the method of transport by creating a library of Universal (UV) Pharmacy Templates that can be used by other Work Groups to derive constrained versions.
Purpose
Background
Historically multiple HL7 Work Groups have developed specifications for pharmacy related content and as a result, there is inconsistency in how medication related content is represented in HL7 V2, V3, CDA and FHIR.
The Pharmacy Work Group often receives questions as to how to model pharmacy related content but in some cases, the use case cannot be met with the existing models.
This Implementation Guide provides a CDA R2 library of pharmacy templates that can be used by HL7 Work Groups or external projects to derive constrained versions of models for pharmacy related content.
Scope
This Pharmacy Templates Implementation Guide defines common pharmacy artifacts (order, dispense, administration and statement) in CDA R2 format. The scope of the Implementation Guide is limited to Medication Order and Medication Statement, Medication Dispense and Medication Administration. The content was developed by aligning and harmonizing the existing specifications for Consolidated CDA (C-CDA Release 2.1[1]).
Ballot Status of the Document
The Implementation Guide was balloted as Standard for Trial Use (STU) and then goes to Normative.
Audience
- Clinical and Public Health laboratories
- Immunization Registries
- Pharmaceutical Vendors
- EHR/PHR vendors
- Clinical Decision Support Systems
- HIS Vendors
- Emergency Services Providers
- Healthcare Institutions
- Pharmacists
- Physicians and other Clinicians
Relationships with other projects and guides
- Consolidated CDA (C-CDA)
- HL7 Version 3 Pharmacy Models
- HL7 FHIR® Pharmacy Resources
- International Patient Summary (IPS), where all pharmacy related templates are specialisations of the corresponding templates defined in this guide
Principles and background
The Pharmacy Work Group has a set of rich set of existing models that were used as the basis for the implementation guide including HL7 V3 models and FHIR resources.
This implementation guide was created by the Pharmacy Work Group using the following approach:
- Review of Consolidated CDA (C-CDA[1]) to identify templates that include pharmacy related content
- Compare C-CDA templates to existing Pharmacy HL7 V3 models and Pharmacy FHIR resources to identify differences and gaps
- Create universal templates to that can be constrained for use for new templates.
For the Medication Model reflected in template 2.16.840.1.113883.10.21.4.11 UV Medication Information (detail), the Common Message Element Type CMET R_Medication Universal” (COCT_MT230100UV02), Release 2 (as published in HL7 V3 2017, V2.0.2 Dec 2010, derived from Common Product Model) was used to construct the CDA extension elements (see also "Extensions" used in this guide in the appendix).
The template rules are formalized using the computable format defined by the HL7 Templates Standard: Specification and Use of Reusable Information Constraint Templates, Release 1[2] in order to facilitate also the automatic generation of consistent testing and validation capabilities.
Technical Background
What is a CDA
CDA R2 is "… a document markup standard that specifies the structure and semantics of clinical documents for the purpose of exchange” [CDA R2, Section 1.1]. Clinical documents, according to CDA, have the following characteristics:
- Persistence
- Stewardship
- Potential for authentication
- Context
- Wholeness
- Human readability
CDA defines a header for classification and management and a document body that carries the clinical record. While the header metadata are prescriptive and designed for consistency across all instances, the body is highly generic, leaving the designation of semantic requirements to implementation.
Templated CDA
CDA R2 can be constrained by mechanisms defined in the “Refinement and Localization” section of the HL7 Version 3 Interoperability Standards. The mechanism most commonly used to constrain CDA is referred to as “templated CDA”. This specification created a set of artifacts containing modular CDA templates (and associated value sets) for the purpose of the International Patient Summary, and the templates can be reused across any number of CDA document types.
There are different kinds of templates that might be created. Among them, the most common ones are:
- CDA Document Level Templates constrain fields in the Clinical Document Architecture (CDA) header, and define containment relationships to CDA sections.
For example, a History-and-Physical document-level template might require that the patient’s name be present, and that the document contain a Physical Exam section.
- CDA Header Level Templates constrain fields for parts of the CDA header, like the patient (record target), the author, participations or the service event.
- CDA Section Level Templates constrain fields in the CDA section, and define containment relationships to CDA entries.
For example, a Physical-exam section-level template might require that the section/code be fixed to a particular LOINC code, and that the section contain a Systolic Blood Pressure observation.
- CDA Entry Level Templates constrain the CDA clinical statement model in accordance with real world observations and acts.
For example, a Systolic-blood-pressure entry-level template defines how the CDA Observation class is constrained (how to populate observation/code, how to populate observation/value, etc.) to represent the notion of a systolic blood pressure.
Open and Closed Templates
Open templates permit anything to be done in the underlying standard that is not explicitly prohibited. This allows templates to be built up over time that extend and go beyond the original use cases for which they were originally designed.
Closed templates only permit what has been defined in the template, and do not permit anything beyond that. There are good reasons to use closed templates, sometimes having to do with local policy. For example, in communicating information from a healthcare provider to an insurance company, some information may need to be omitted to ensure patient privacy laws are followed.
Most templates developed for CDA are of the open sort.
Template versioning
Template versioning is needed to enable template designs to evolve over time.
Template versioning enables template designers to control and shape the conformance statements that make up a template’s design over time tailoring the design to fit the template’s intended purpose.
Each template version is associated with a particular template. The template – as a whole – has a mandatory globally unique, non-semantic, identifier. The identifier serves as the identifier of the original intent of the template and as the identifier of the set of versions that represent the template over time.
Template versions have a mandatory timestamp (date and optional time), called the “effective date”. The date can be seen as the point in time when the template version “came into being”, i.e. was recognized as existent by the governance group. Use of the template prior to this date would be considered an invalid use of the template.
For further information on Templates, Template Versions and related topics refer to the HL7 Templates Standard[2].
Identifiers for Templates and Value Sets
This specification specifies CDA Entry Level Templates only. They can be re-used in any appropriate context, such as an Entry of a medication section.
Two "root" Entry Templates are provided as entry points for the four described use cases:
- UV Medication Order (2.16.840.1.113883.10.21.4.1)
- UV Medication Statement (2.16.840.1.113883.10.21.4.7)
- UV Medication Administration (2.16.840.1.113883.10.21.4.13)
- UV Medication Dispense (2.16.840.1.113883.10.21.4.15)
These templates use other Entry Level Templates that are all listed in a subsequent section of this document.
This specification uses the following OIDs for the artifacts that are registered at the HL7 OID registry.
- The root OID for templates is 2.16.840.1.113883.10.21
- Entry Level templates are summarized under 2.16.840.1.113883.10.21.4, e.g. 2.16.840.1.113883.10.21.4.5 UV Substitution Permission
- “other” assistance templates are summarized under 2.16.840.1.113883.10.21.9, e.g. 2.16.840.1.113883.10.22.9.1 UV Use Period
- The root OID for Value Sets is 2.16.840.1.113883.11
The sub branches for templates follow the recommendations of HL7 International and ISO 13582[3].
Namespace Identifier
The CDA extensions for Pharmacy defined by the Pharmacy Workgroup are handled under the XML namespace identifier
urn:hl7-org:pharm
and typically use the namespace prefix
pharm:
How to read this document
All artifacts (templates, value sets etc.) listed with the status  Draft or
Draft or  Pending are subject to ballot comments.
Pending are subject to ballot comments.
Artifacts with other status information, especially  Final or Active are not (directly) part of the ballot and some artifacts actually even come from external sources. Reference artifacts are indicated by the symbol
Final or Active are not (directly) part of the ballot and some artifacts actually even come from external sources. Reference artifacts are indicated by the symbol
ref
. These reference artifacts are also not subject to the ballot, as they might be balloted elsewhere already.
The PDF version contains a ruler on the left side of the pages. A ruler has the page number on top of it and allows locating a line at the page by simply specifying the number at the scale tick. This is more precise and allows also commenting on graphics and pictures.
For example if you have a comment on page 29 because of a typo (see figure), you simply specify the error with its location p0029-04.
Of course you can also refer by classical chapter and section numbers. The use of the ruler has the ballot team's preference, though.

[Figure 1] To locate a typo on page 29 as a ballot comment, simply specify the location p0029-04.
Reading Publication Artifacts
A reading guide is available that explains the formalisms used to express the publication artifacts, i.e. template meta data and template design. For convenience the guide is included in the appendix.
Functional requirements and high-level use cases
The following use cases are relevant to the pharmacy domain for both community and institutional settings:
- Prescribing a medication (aka Prescription or Order or Request)
- Dispensing a medication
- Recording the administration of a medication
- Recording the use of a medication (in the past, current or future)
The following definitions are relevant to this Implementation Guide:
- Prescribing is an activity that can be performed by a variety of healthcare professionals and involves a variety of orderable items (see glossary entry). For the purposes of the following Implementation Guide, prescribing is defined as the act of authorizing the usage of a medication in various settings for example, inpatient, community, and long term care. This could include initiating a new medication order or making all kinds of modifications to existing orders.
- Dispensing is the provision of a medication or other material to a caregiver in fulfillment of a prescription or medication order. It supplies the materials needed to perform the prescribed actions by those who will perform them. Examples of dispensing include eyeglasses, contact lenses and medications. Dispensing is defined as supplying a medication in fulfilment of a prescription or medication order. While dispensing is usually performed by a pharmacist, other health care providers such as nurses or physicians may also dispense.
- Administration is an activity undertaken to give medication to the patient. In the community, this process is usually not recorded, since the majority occurs in the patient's home; only administrations undertaken by a healthcare professional, such as vaccination, tend to be formally documented. Administration of medication in the institutional setting is usually recorded on a dose-by-dose basis, and may be messaged on that basis, or a summary of all the administrations occurring during an inpatient stay may be described.
- Medication Statement is an activity that can be performed by a variety of healthcare professionals, or the patient, or non-healthcare professionals. Examples of recording medication statements include taking a patient's medication history, recording reported use of medications where the source of the patient information is from a third party and not the patient e.g. a family member when the patient is unable to communicate their medication history.
Templates
Use Case Entry Level Templates
As mentioned before, this specification defines two "root" Entry Level Templates, one for each of the covered use cases. All entry templates are used in the context of a CDA section.
UV Medication Order
The following graph gives an overview of the high-level template components of this template, followed by the actual definition.
Note: If you need to include multiple ordered medications as part of a single order, you can include multiple CDA entries under one CDA section. CDA Section definitions are not part of this guide.
-
Entry UV Medication Order (2.16.840.1.113883.10.21.4.1)
-
Entry UV Use Period (2.16.840.1.113883.10.21.9.1)
-
Entry CDA Subject (Body) (2.16.840.1.113883.10.12.320)
-
Entry UV Medication Information (simple) (2.16.840.1.113883.10.21.4.10)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry UV Medication Information (detail) (2.16.840.1.113883.10.21.4.11)
-
Entry UV Content (2.16.840.1.113883.10.21.4.17)
-
Entry UV Generalized Medicine Class (2.16.840.1.113883.10.21.4.19)
-
Entry UV Content (2.16.840.1.113883.10.21.4.17)
-
Entry UV Generalized Medicine Class (2.16.840.1.113883.10.21.4.19)
-
Entry UV Content (2.16.840.1.113883.10.21.4.17)
-
Entry UV Generalized Medicine Class (2.16.840.1.113883.10.21.4.19)
-
Entry UV Content (2.16.840.1.113883.10.21.4.17)
-
Entry UV Generalized Medicine Class (2.16.840.1.113883.10.21.4.19)
-
Entry UV Ingredient (2.16.840.1.113883.10.21.4.18)
-
Entry UV Ingredient (2.16.840.1.113883.10.21.4.18)
-
Entry UV Ingredient (2.16.840.1.113883.10.21.4.18)
-
Entry UV Ingredient (2.16.840.1.113883.10.21.4.18)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA Author (Body) (2.16.840.1.113883.10.12.318)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA Participant (Body) (2.16.840.1.113883.10.12.321)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
Entry CDA PlayingEntity (2.16.840.1.113883.10.12.313)
-
Entry CDA Participant (Body) (2.16.840.1.113883.10.12.321)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
Entry CDA PlayingEntity (2.16.840.1.113883.10.12.313)
-
Entry UV Subordinate Substance Administration (2.16.840.1.113883.10.21.4.6)
-
Entry UV Dispense Request (2.16.840.1.113883.10.21.4.2)
-
Entry CDA Subject (Body) (2.16.840.1.113883.10.12.320)
-
Entry CDA ManufacturedProduct (2.16.840.1.113883.10.12.312)
-
Entry CDA LabeledDrug (2.16.840.1.113883.10.12.310)
-
Entry CDA Material (2.16.840.1.113883.10.12.311)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA Performer (Body) (2.16.840.1.113883.10.12.323)
-
* CDA AssignedEntity (2.16.840.1.113883.10.12.153)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA Participant (Body) (2.16.840.1.113883.10.12.321)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
Entry CDA PlayingEntity (2.16.840.1.113883.10.12.313)
-
Entry CDA Participant (Body) (2.16.840.1.113883.10.12.321)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
Entry CDA PlayingEntity (2.16.840.1.113883.10.12.313)
-
Entry CDA Participant (Body) (2.16.840.1.113883.10.12.321)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
Entry CDA PlayingEntity (2.16.840.1.113883.10.12.313)
-
Entry CDA Participant (Body) (2.16.840.1.113883.10.12.321)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
Entry CDA PlayingEntity (2.16.840.1.113883.10.12.313)
-
Entry UV ClinicalStatement Observation (2.16.840.1.113883.10.21.4.3)
-
Entry CDA Subject (Body) (2.16.840.1.113883.10.12.320)
-
Entry CDA Specimen (2.16.840.1.113883.10.12.322)
-
Entry CDA Performer (Body) (2.16.840.1.113883.10.12.323)
-
* CDA AssignedEntity (2.16.840.1.113883.10.12.153)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA Author (Body) (2.16.840.1.113883.10.12.318)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA Informant (Body) (2.16.840.1.113883.10.12.319)
-
* CDA AssignedEntity (2.16.840.1.113883.10.12.153)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA RelatedEntity (2.16.840.1.113883.10.12.316)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
Entry CDA Participant (Body) (2.16.840.1.113883.10.12.321)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
Entry CDA PlayingEntity (2.16.840.1.113883.10.12.313)
-
Entry CDA Reference (2.16.840.1.113883.10.12.324)
-
Entry CDA ExternalAct (2.16.840.1.113883.10.12.325)
-
Entry CDA ExternalObservation (2.16.840.1.113883.10.12.326)
-
Entry CDA ExternalProcedure (2.16.840.1.113883.10.12.327)
-
Entry CDA ExternalDocument (2.16.840.1.113883.10.12.328)
-
Entry CDA Precondition (2.16.840.1.113883.10.12.329)
-
Entry IPS Internal Reference (2.16.840.1.113883.10.22.4.31)
-
Entry UV ClinicalStatement Observation (2.16.840.1.113883.10.21.4.3)
-
Entry CDA Subject (Body) (2.16.840.1.113883.10.12.320)
-
Entry CDA Specimen (2.16.840.1.113883.10.12.322)
-
Entry CDA Performer (Body) (2.16.840.1.113883.10.12.323)
-
* CDA AssignedEntity (2.16.840.1.113883.10.12.153)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA Author (Body) (2.16.840.1.113883.10.12.318)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA Informant (Body) (2.16.840.1.113883.10.12.319)
-
* CDA AssignedEntity (2.16.840.1.113883.10.12.153)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA RelatedEntity (2.16.840.1.113883.10.12.316)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
Entry CDA Participant (Body) (2.16.840.1.113883.10.12.321)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
Entry CDA PlayingEntity (2.16.840.1.113883.10.12.313)
-
Entry CDA Reference (2.16.840.1.113883.10.12.324)
-
Entry CDA ExternalAct (2.16.840.1.113883.10.12.325)
-
Entry CDA ExternalObservation (2.16.840.1.113883.10.12.326)
-
Entry CDA ExternalProcedure (2.16.840.1.113883.10.12.327)
-
Entry CDA ExternalDocument (2.16.840.1.113883.10.12.328)
-
Entry CDA Precondition (2.16.840.1.113883.10.12.329)
-
Entry UV Substitution Permission (2.16.840.1.113883.10.21.4.5)
-
Entry UV ClinicalStatement Encounter (2.16.840.1.113883.10.21.4.4)
-
Entry UV Comment Activity (2.16.840.1.113883.10.21.4.12)
-
Entry CDA Author (Body) (2.16.840.1.113883.10.12.318)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA Precondition (2.16.840.1.113883.10.12.329)
|
The boxes reflect the CDA Template Types. Symbols: * denotes templates with more than one classification, @ indicates a recursion in the definition
| Id | 2.16.840.1.113883.10.21.4.1 | Effective Date | 2023‑01‑31 11:29:28 |
|---|
| Status |  Draft Draft | Version Label | 2023 |
|---|
| Name | UVSubstanceadministrationrequest | Display Name | UV Medication Order |
|---|
| Description | Universal Medication Order (Substance Administration Request) |
|---|
| Context | Parent nodes of template element with id 2.16.840.1.113883.10.21.4.1 |
|---|
| Label | MedicationOrder
|
|---|
| Classification | CDA Entry Level Template |
|---|
| Open/Closed | Open (other than defined elements are allowed) |
|---|
| Uses | | Uses 14 templates | | Uses | as | Name | Version |
|---|
| 2.16.840.1.113883.10.21.9.1 | Include |  UV Use Period (2023) UV Use Period (2023) | DYNAMIC | | 2.16.840.1.113883.10.12.320 | Containment |  CDA Subject (Body) CDA Subject (Body) | DYNAMIC | | 2.16.840.1.113883.10.21.4.10 | Containment |  UV Medication Information (simple) (R1-STU2-ballot) UV Medication Information (simple) (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.21.4.11 | Containment |  UV Medication Information (detail) (2023) UV Medication Information (detail) (2023) | DYNAMIC | | 2.16.840.1.113883.10.12.318 | Containment |  CDA Author (Body) CDA Author (Body) | DYNAMIC | | 2.16.840.1.113883.10.12.321 | Containment |  CDA Participant (Body) CDA Participant (Body) | DYNAMIC | | 2.16.840.1.113883.10.21.4.6 | Containment |  UV Subordinate Substance Administration (2023) UV Subordinate Substance Administration (2023) | DYNAMIC | | 2.16.840.1.113883.10.21.4.2 | Containment |  UV Dispense Request (R1-STU2-ballot) UV Dispense Request (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.21.4.3 | Containment |  UV ClinicalStatement Observation (R1-STU2-ballot) UV ClinicalStatement Observation (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.22.4.31 | Containment |  IPS Internal Reference (STU1) IPS Internal Reference (STU1) | DYNAMIC | | 2.16.840.1.113883.10.21.4.5 | Containment |  UV Substitution Permission (R1-STU2-ballot) UV Substitution Permission (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.21.4.4 | Containment |  UV ClinicalStatement Encounter (R1-STU2-ballot) UV ClinicalStatement Encounter (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.21.4.12 | Containment |  UV Comment Activity (R1-STU2-ballot) UV Comment Activity (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.12.329 | Containment |  CDA Precondition CDA Precondition | DYNAMIC |
|
|
|---|
| Relationship | Version: template 2.16.840.1.113883.10.21.4.1 UV Medication Order (2015‑10‑07)
Specialization: template 2.16.840.1.113883.10.12.308 CDA SubstanceAdministration (2005‑09‑07) ref ad1bbr- |
|---|
| Example | | Example | <substanceAdministration classCode="SBADM" moodCode="RQO">
<templateId root="2.16.840.1.113883.10.21.4.1"/> <id root="1.2.3.99.99.99" extension="58768437489739"/> <code code="..." codeSystem="..."/> <text>...</text> <statusCode code="active"/> <effectiveTime value="..."/> <repeatNumber value="..."/> <routeCode code="IPINHL" codeSystem="2.16.840.1.113883.5.112" displayName="Inhalation, respiratory Inhalation, intrapulmonary Inhalation, oral"/> <approachSiteCode code="..." codeSystem="2.16.840.1.113883.5.1052"/> <administrationUnitCode code="PUFF" codeSystem="2.16.840.1.113883.5.85" displayName="Puff"/> <consumable typeCode="CSM">
<!-- Consumable -->
</consumable> <participant typeCode="DEV">
<!-- Device -->
</participant> <participant typeCode="LOC">
<!-- Location -->
</participant> <entryRelationship typeCode="COMP">
<!-- Subordinate Substance Administrations -->
</entryRelationship> <entryRelationship typeCode="COMP">
<!-- Annotations -->
</entryRelationship> <precondition>
<!-- Precondition -->
</precondition></substanceAdministration> |
|
|---|
| Item | DT | Card | Conf | Description | Label |
|---|
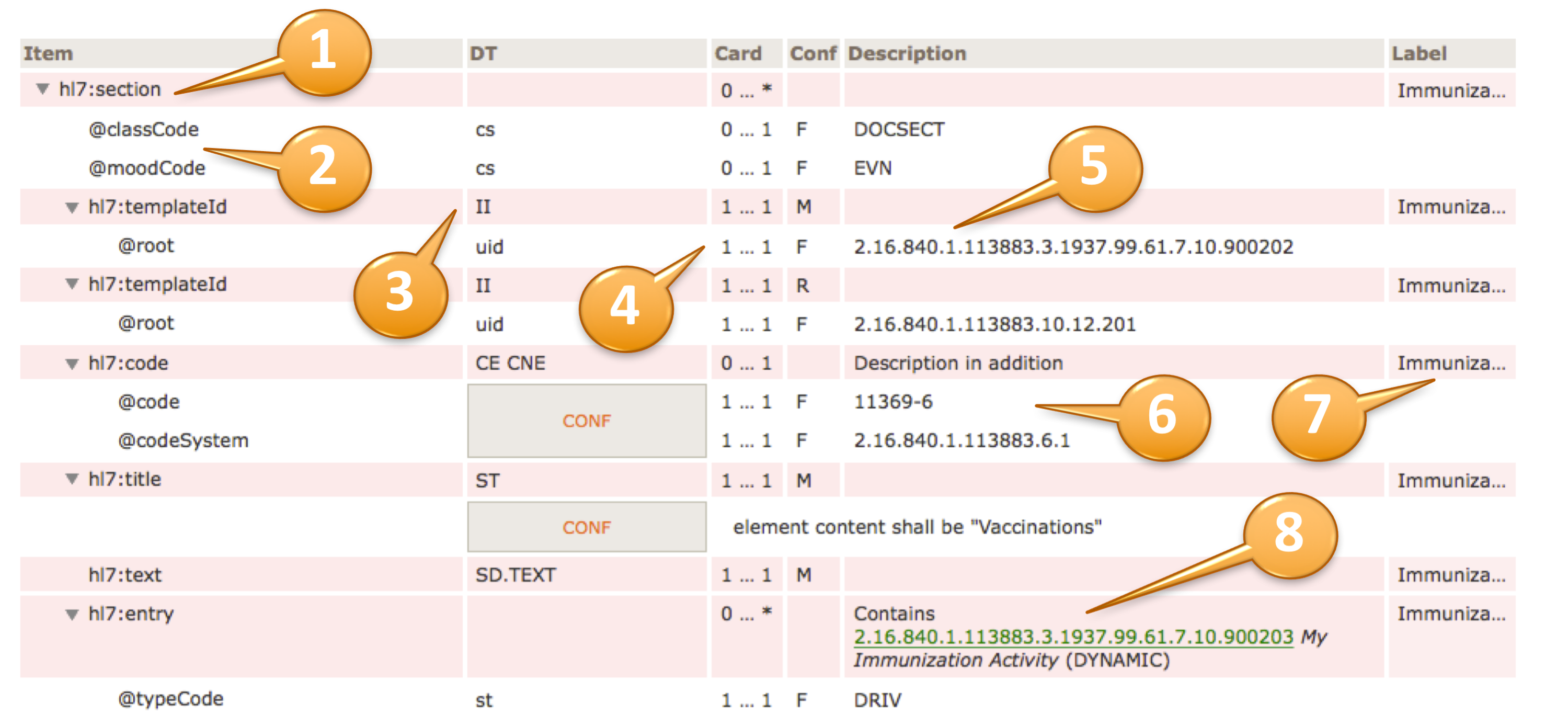
hl7:substanceAdministration
|
| | | | | Medi...rder |  | @classCode
|
| cs | 1 … 1 | F | SBADM |  | @moodCode
|
| cs | 1 … 1 | F | RQO |  | hl7:templateId
|
| II | 1 … 1 | M | | Medi...rder |  |  | @root
|
| uid | 1 … 1 | F | 2.16.840.1.113883.10.21.4.1 |  | hl7:id
|
| II | 1 … * | R | | Medi...rder |  | hl7:code
|
| CD (extensible) | 0 … 1 | R | | Medi...rder | | | CONF | | The value of @code should be drawn from value set 2.16.840.1.113883.1.11.19708 ActSubstanceAdministrationCode (DYNAMIC) |
|  | hl7:text
|
| ED | 0 … 1 | | | Medi...rder |  | hl7:statusCode
|
| CS (required) | 1 … 1 | M | | Medi...rder | | | CONF | | The value of @code shall be drawn from value set 2.16.840.1.113883.11.21.2 ActStatusActiveCompletedAbortedSuspended (DYNAMIC) |
| | Included | | | from 2.16.840.1.113883.10.21.9.1 UV Use Period (DYNAMIC) | | Choice | 1 … 1 | | The effectiveTime element encodes the use period of the medication, it is always expressed as an interval of time.
It may be expressed using the low and high OR with the width element.
The first is used to indicate a specified interval (e.g. from march 15th, 2017); the latter for indicating a 'floating' period (e.g. 2 weeks). Elements to choose from:- hl7:effectiveTime[hl7:low | hl7:high][not(hl7:width)]
- hl7:effectiveTime[hl7:width][not(hl7:low|hl7:high)]
- hl7:effectiveTime[hl7:low | hl7:width][not(hl7:high)]
|  |  | hl7:effectiveTime
|
| IVL_TS | 0 … 1 | C | Case 1: specified interval
The low and high values of the first effectiveTime element represent the start and stop times for the medication. The low value represents the start time, and the high value represents the stop time. If either the low or the high value is unknown, this shall be recorded by setting the nullFlavor attribute to UNK.
In case of unbounded period (continuous therapy) the high element will be valued with the nullFlavor attribute to NA.
The high value records the end of the medication regime according to the information provided in the prescription or order. For example, if the prescription is for enough medication to last 30 days, then the high value should contain a date that is 30 days later then the low value. The rationale is that a provider, seeing a prescription that has not been refilled would normally assume that the medication is no longer being taken, even if the intent of the treatment plan is to continue the medication indefinitely. | Medi...rder | where [hl7:low or
[not(hl7:width)] | | | cs | 0 … 1 | | | | | Example | Known Interval <effectiveTime type="IVL_TS">
<low value="20130321"/> <high value="20140321"/></effectiveTime> | | | Example | Information not available about the period <effectiveTime type="IVL_TS" nullFlavor="NI"/> | | | Example | Unknown end date <effectiveTime type="IVL_TS">
<low value="20130321"/> <high nullFlavor="UNK"/></effectiveTime> | | | Example | continous therapy <effectiveTime type="IVL_TS">
<low value="20130321"/> <high nullFlavor="NA"/></effectiveTime> | | IVXB_TS | 1 … 1 | R | | Medi...rder | | IVXB_TS | 0 … 1 | R | | Medi...rder |  |  | hl7:effectiveTime
|
| IVL_TS | 0 … 1 | C | Case 2: 'floating' period:
The width element is used to specify a period of (actual or intended) administration that is not anchored to any specific date (e.g. a two weeks therapy) | Medi...rder | where [hl7:width] [not(hl7:lowor
hl7:high)] | | | | Example | 2 week period <effectiveTime type="IVL_TS">
<width value="2" unit="w"/></effectiveTime> | | | | NP | | Medi...rder | | | | NP | | Medi...rder | | | | NP | | Medi...rder | | PQ | 1 … 1 | R | | Medi...rder | | cs | 1 … 1 | R | | | | CONF | | The value of @unit shall be drawn from value set 2.16.840.1.113883.11.21.1 Medication Time Units (UCUM) (DYNAMIC) |
|  |  | hl7:effectiveTime
|
| IVL_TS | 0 … 1 | C | Case 3: anchored period:
The width element is used to specify a period of (actual or intended) administration anchored to a specific date (e.g. a two weeks therapy starting today) | Medi...rder | where [hl7:low or
[not(hl7:high)] | | | | Example | 2 week period starting on 2013-03-21 <effectiveTime type="IVL_TS">
<low value="20130321"/> <width value="2" unit="w"/></effectiveTime> | | IVXB_TS | 0 … 1 | C | | Medi...rder | | PQ | 1 … 1 | R | | Medi...rder | | cs | 1 … 1 | R | | | | CONF | | The value of @unit shall be drawn from value set 2.16.840.1.113883.11.21.1 Medication Time Units (UCUM) (DYNAMIC) |
|  | hl7:repeatNumber
|
| IVL_INT | 0 … 1 | | | Medi...rder |  | hl7:routeCode
|
| CE (example) | 0 … 1 | | | Medi...rder | | | CONF | | Examples of the value of @code are in the value set 2.16.840.1.113883.1.11.14581 RouteOfAdministration (DYNAMIC) |
|  | hl7:approachSiteCode
|
| CD (example) | 0 … * | | | Medi...rder | | | CONF | | Examples of the value of @code are in the value set 2.16.840.1.113883.1.11.19724 HumanSubstanceAdministrationSite (DYNAMIC) |
|  | hl7:doseQuantity
|
| IVL_PQ | | NP | | Medi...rder |  | hl7:rateQuantity
|
| IVL_PQ | | NP | | Medi...rder |  | hl7:maxDoseQuantity
|
| RTO_PQ_PQ | 0 … 1 | | | Medi...rder |  | hl7:administrationUnitCode
|
| CE | | NP | | Medi...rder |  | hl7:subject
|
| | 0 … 1 | C | The patient, subject to requested dispenses or subject to substances being administered to.
Contains 2.16.840.1.113883.10.12.320 CDA Subject (Body) (DYNAMIC) | Medi...rder | | | Constraint | Condition: This can be omitted if the patient context that is provided in the CDA header is identical to the subject | | Choice | 1 … 1 | | Elements to choose from:- hl7:consumable containing template 2.16.840.1.113883.10.21.4.10 UV Medication Information (simple) (DYNAMIC)
- hl7:consumable containing template 2.16.840.1.113883.10.21.4.11 UV Medication Information (detail) (DYNAMIC)
|  |  | hl7:consumable
|
| | 0 … 1 | R | Consumable: The medication that is administered (simple)
Contains 2.16.840.1.113883.10.21.4.10 UV Medication Information (simple) (DYNAMIC) | Medi...rder | | cs | 1 … 1 | F | CSM |  |  | hl7:consumable
|
| | 0 … 1 | R | Consumable: The medication that is administered (detail)
Contains 2.16.840.1.113883.10.21.4.11 UV Medication Information (detail) (DYNAMIC) | Medi...rder | | cs | 1 … 1 | F | CSM |  | hl7:author
|
| | 0 … * | | Prescriber: A party that originates the order and therefore has responsibility for the information given in the order.
Contains 2.16.840.1.113883.10.12.318 CDA Author (Body) (DYNAMIC) | Medi...rder |  | hl7:participant
|
| | 0 … 1 | | Record Target: indicates the person who's medical record holds the documentation of this medication statement. This element is only populated when the document is placed in a medical record of someone other than the patient (subject).
Contains 2.16.840.1.113883.10.12.321 CDA Participant (Body) (DYNAMIC) | Medi...rder | | where [@typeCode='RCT'] | |  |  | @typeCode
|
| cs | 1 … 1 | F | RCT |  | hl7:participant
|
| | 0 … 1 | | Verifier: The person or organization that has primary responsibility for the order. The responsible party is not necessarily present in an action, but is accountable for the action through the power to delegate.
Contains 2.16.840.1.113883.10.12.321 CDA Participant (Body) (DYNAMIC) | Medi...rder | | where [@typeCode='VRF'] | |  |  | @typeCode
|
| cs | 1 … 1 | F | VRF |  | hl7:entryRelationship
|
| | 0 … * | C | Subordinate Substance Administration Request as a component of the overall order.
At least one subordinated Substance Administration should be present to convey information about dosages (dose, frequency of intakes,..) unless dosage is unknown.
Subordinated Substance Administration elements can be also used either to handle split dosing, or to support combination medications.
Contains 2.16.840.1.113883.10.21.4.6 UV Subordinate Substance Administration (DYNAMIC) | Medi...rder | | where [hl7:substanceAdministration] | |  |  | @typeCode
|
| cs | 1 … 1 | F | COMP | | | Constraint | At least one subordinate element SHALL be present. | | | Example | <entryRelationship typeCode="COMP">
<!-- component: Subordinate Substance Administration Request. -->
<substanceAdministration classCode="SBADM" moodCode="RQO">
<templateId root="2.16.840.1.113883.10.21.4.6"/> <!-- .. -->
</substanceAdministration></entryRelationship> |  |  | hl7:sequenceNumber
|
| INT | 0 … 1 | | Sequence number of the Subordinate Substance Administration. | Medi...rder |  | hl7:entryRelationship
|
| | 0 … 1 | R | Dispense Request as a component of the overall order. This element is used in the medication order when the dispense request information contains additional information to support a fully specified medication prescription. For example, to include the validity period of the dispense or the organization to dispense the medication.
Contains 2.16.840.1.113883.10.21.4.2 UV Dispense Request (DYNAMIC) | Medi...rder | | where [hl7:supply] | |  |  | @typeCode
|
| cs | 1 … 1 | F | COMP | | | Example | <entryRelationship typeCode="COMP">
<!-- component: The Dispense Request is a component of the overall order. -->
<supply classCode="SPLY" moodCode="RQO">
<templateId root="2.16.840.1.113883.10.21.4.2"/> <!-- .. -->
</supply></entryRelationship> | | Choice | 0 … * | | Elements to choose from:- hl7:entryRelationship containing template 2.16.840.1.113883.10.21.4.3 UV ClinicalStatement Observation (DYNAMIC)
- hl7:entryRelationship containing template 2.16.840.1.113883.10.22.4.31 IPS Internal Reference (DYNAMIC)
|  |  | hl7:entryRelationship
|
| | 0 … * | R | Reason: Specifies the reason (indication) for authoring the order.
Contains 2.16.840.1.113883.10.21.4.3 UV ClinicalStatement Observation (DYNAMIC) | Medi...rder | | cs | 1 … 1 | F | RSON | | | Example | <hl7:entryRelationship typeCode="RSON">
<priorityNumber value="1"/> <!-- template 2.16.840.1.113883.10.21.4.3 'UV ClinicalStatement Observation' (2016-05-01T00:00:00) -->
</hl7:entryRelationship> | | INT.NONNEG | 0 … 1 | R | Indicates the priority of this reason for the order in relation to its sibling reasons. | Medi...rder |  |  | hl7:entryRelationship
|
| | 0 … * | R | Reason: Specifies the reason (indication) for authoring the order.
Contains 2.16.840.1.113883.10.22.4.31 IPS Internal Reference (DYNAMIC) | Medi...rder | | cs | 1 … 1 | F | RSON | | | Example | <entryRelationship typeCode="RSON">
<priorityNumber value="1"/> <act>
<!-- Clinical Statement Minimal -->
</act></entryRelationship> | | INT.NONNEG | 0 … 1 | R | Indicates the priority of this reason for the order in relation to its sibling reasons. | Medi...rder |  | hl7:entryRelationship
|
| | 0 … * | R | Pertinent Information: Specifies any pertinent information (observation) relevant to the order.
Contains 2.16.840.1.113883.10.21.4.3 UV ClinicalStatement Observation (DYNAMIC) | Medi...rder |  |  | @typeCode
|
| cs | 1 … 1 | F | PERT | | | Example | <entryRelationship typeCode="PERT">
<observation>
<!-- Clinical Statement Observation -->
</observation></entryRelationship> |  | hl7:entryRelationship
|
| | 0 … 1 | R | Permission: The order can be the subject of the permissions related to substitution.
Contains 2.16.840.1.113883.10.21.4.5 UV Substitution Permission (DYNAMIC) | Medi...rder | | where [hl7:act] | |  |  | @typeCode
|
| cs | 1 … 1 | F | COMP |  | hl7:entryRelationship
|
| | 0 … 1 | R | Encounter: Used to link an order to a specific encounter.
Contains 2.16.840.1.113883.10.21.4.4 UV ClinicalStatement Encounter (DYNAMIC) | Medi...rder | | where [hl7:encounter] | |  |  | @typeCode
|
| cs | 1 … 1 | F | COMP | | | Example | <encounter classCode="ENC" moodCode="EVN">
<id/> <code code="..."/></encounter> |  | hl7:entryRelationship
|
| | 0 … * | | Annotations: The Medication Order can be the subject of annotations.
Contains 2.16.840.1.113883.10.21.4.12 UV Comment Activity (DYNAMIC) | Medi...rder |  |  | @typeCode
|
| cs | 1 … 1 | F | COMP |  | hl7:precondition
|
| | 0 … * | | Precondition: A requirement to be true before the SubstanceAdministration is performed.
Contains 2.16.840.1.113883.10.12.329 CDA Precondition (DYNAMIC) | Medi...rder |
|
UV Medication Statement
The following graph gives an overview of the high-level template components of this template, followed by the actual definition.
-
Entry UV Medication Statement (2.16.840.1.113883.10.21.4.7)
-
Entry UV Use Period (2.16.840.1.113883.10.21.9.1)
-
Entry CDA Subject (Body) (2.16.840.1.113883.10.12.320)
-
Entry UV Medication Information (simple) (2.16.840.1.113883.10.21.4.10)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry UV Medication Information (detail) (2.16.840.1.113883.10.21.4.11)
-
Entry UV Content (2.16.840.1.113883.10.21.4.17)
-
Entry UV Generalized Medicine Class (2.16.840.1.113883.10.21.4.19)
-
Entry UV Content (2.16.840.1.113883.10.21.4.17)
-
Entry UV Generalized Medicine Class (2.16.840.1.113883.10.21.4.19)
-
Entry UV Content (2.16.840.1.113883.10.21.4.17)
-
Entry UV Generalized Medicine Class (2.16.840.1.113883.10.21.4.19)
-
Entry UV Content (2.16.840.1.113883.10.21.4.17)
-
Entry UV Generalized Medicine Class (2.16.840.1.113883.10.21.4.19)
-
Entry UV Ingredient (2.16.840.1.113883.10.21.4.18)
-
Entry UV Ingredient (2.16.840.1.113883.10.21.4.18)
-
Entry UV Ingredient (2.16.840.1.113883.10.21.4.18)
-
Entry UV Ingredient (2.16.840.1.113883.10.21.4.18)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA Author (Body) (2.16.840.1.113883.10.12.318)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
* CDA AssignedEntity (2.16.840.1.113883.10.12.153)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA RelatedEntity (2.16.840.1.113883.10.12.316)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
Entry CDA Participant (Body) (2.16.840.1.113883.10.12.321)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
Entry CDA PlayingEntity (2.16.840.1.113883.10.12.313)
-
Entry CDA Participant (Body) (2.16.840.1.113883.10.12.321)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
Entry CDA PlayingEntity (2.16.840.1.113883.10.12.313)
-
Entry UV Subordinate Substance Administration (2.16.840.1.113883.10.21.4.6)
-
Entry UV Medication Order Reference (2.16.840.1.113883.10.21.4.8)
-
Entry CDA Author (Body) (2.16.840.1.113883.10.12.318)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry UV Dispense Event Reference (2.16.840.1.113883.10.21.4.9)
-
Entry UV ClinicalStatement Observation (2.16.840.1.113883.10.21.4.3)
-
Entry CDA Subject (Body) (2.16.840.1.113883.10.12.320)
-
Entry CDA Specimen (2.16.840.1.113883.10.12.322)
-
Entry CDA Performer (Body) (2.16.840.1.113883.10.12.323)
-
* CDA AssignedEntity (2.16.840.1.113883.10.12.153)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA Author (Body) (2.16.840.1.113883.10.12.318)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA Informant (Body) (2.16.840.1.113883.10.12.319)
-
* CDA AssignedEntity (2.16.840.1.113883.10.12.153)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA RelatedEntity (2.16.840.1.113883.10.12.316)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
Entry CDA Participant (Body) (2.16.840.1.113883.10.12.321)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
Entry CDA PlayingEntity (2.16.840.1.113883.10.12.313)
-
Entry CDA Reference (2.16.840.1.113883.10.12.324)
-
Entry CDA ExternalAct (2.16.840.1.113883.10.12.325)
-
Entry CDA ExternalObservation (2.16.840.1.113883.10.12.326)
-
Entry CDA ExternalProcedure (2.16.840.1.113883.10.12.327)
-
Entry CDA ExternalDocument (2.16.840.1.113883.10.12.328)
-
Entry CDA Precondition (2.16.840.1.113883.10.12.329)
-
Entry IPS Internal Reference (2.16.840.1.113883.10.22.4.31)
|
The boxes reflect the CDA Template Types. Symbols: * denotes templates with more than one classification, @ indicates a recursion in the definition
| Id | 2.16.840.1.113883.10.21.4.7 | Effective Date | 2023‑01‑30 08:32:34Other versions this id:  UVMedicationstatement as of 2021‑08‑04 14:09:15 UVMedicationstatement as of 2021‑08‑04 14:09:15 UVMedicationstatement as of 2017‑05‑01 UVMedicationstatement as of 2017‑05‑01
|
|---|
| Status |  Draft Draft | Version Label | 2023 |
|---|
| Name | UVMedicationstatement | Display Name | UV Medication Statement |
|---|
| Description | Universal Medication Statement: Recording a "medication statement" is an activity that can be performed by a variety of healthcare professionals, or the patient, or non-healthcare professionals. Examples of recording medication statements include taking a patient's medication history, recording reported use of medications where the source of the patient
information is from a third party and not the patient e.g. a family member when the patient is unable to communicate their medication history. |
|
| Context | Parent nodes of template element with id 2.16.840.1.113883.10.21.4.7 |
|---|
| Label | MedicationStatement
|
|---|
| Classification | CDA Entry Level Template |
|---|
| Open/Closed | Open (other than defined elements are allowed) |
|---|
| Uses | | Uses 13 templates | | Uses | as | Name | Version |
|---|
| 2.16.840.1.113883.10.21.9.1 | Include |  UV Use Period (2023) UV Use Period (2023) | DYNAMIC | | 2.16.840.1.113883.10.12.320 | Containment |  CDA Subject (Body) CDA Subject (Body) | DYNAMIC | | 2.16.840.1.113883.10.21.4.10 | Containment |  UV Medication Information (simple) (R1-STU2-ballot) UV Medication Information (simple) (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.21.4.11 | Containment |  UV Medication Information (detail) (2023) UV Medication Information (detail) (2023) | DYNAMIC | | 2.16.840.1.113883.10.12.318 | Containment |  CDA Author (Body) CDA Author (Body) | DYNAMIC | | 2.16.840.1.113883.10.12.153 | Containment |  CDA AssignedEntity CDA AssignedEntity | DYNAMIC | | 2.16.840.1.113883.10.12.316 | Containment |  CDA RelatedEntity CDA RelatedEntity | DYNAMIC | | 2.16.840.1.113883.10.12.321 | Containment |  CDA Participant (Body) CDA Participant (Body) | DYNAMIC | | 2.16.840.1.113883.10.21.4.6 | Containment |  UV Subordinate Substance Administration (2023) UV Subordinate Substance Administration (2023) | DYNAMIC | | 2.16.840.1.113883.10.21.4.8 | Containment |  UV Medication Order Reference (R1-STU2-ballot) UV Medication Order Reference (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.21.4.9 | Containment |  UV Dispense Event Reference (R1-STU2-ballot) UV Dispense Event Reference (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.21.4.3 | Containment |  UV ClinicalStatement Observation (R1-STU2-ballot) UV ClinicalStatement Observation (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.22.4.31 | Containment |  IPS Internal Reference (STU1) IPS Internal Reference (STU1) | DYNAMIC |
|
|
|---|
| Relationship | Version: template 2.16.840.1.113883.10.21.4.7 UV Medication Statement (2021‑08‑04 14:09:15)
Version: template 2.16.840.1.113883.10.21.4.7 UV Medication Statement (2017‑05‑01)
Specialization: template 2.16.840.1.113883.10.12.308 CDA SubstanceAdministration (2005‑09‑07) ref ad1bbr- |
|---|
| Example | | Example | <substanceAdministration classCode="SBADM" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.21.4.7"/> <id root="1.2.3.99.99.99" extension="988437489739"/> <code code="..." codeSystem="..."/> <text>...</text> <statusCode code="active"/> <effectiveTime value="..."/> <repeatNumber value="..."/> <routeCode code="SOAK" codeSystem="2.16.840.1.113883.5.112" displayName="Immersion (soak)"/> <approachSiteCode code="..." codeSystem="2.16.840.1.113883.5.1052"/> <administrationUnitCode code="PUFF" displayName="Puff" codeSystem="2.16.840.1.113883.5.85"/> <consumable typeCode="CSM">
<!-- Consumable -->
</consumable> <participant typeCode="DEV">
<!-- Device -->
</participant> <participant typeCode="LOC">
<!-- Location -->
</participant> <entryRelationship typeCode="COMP">
<!-- Subordinate Substance Administrations -->
</entryRelationship> <entryRelationship typeCode="COMP">
<!-- Annotations -->
</entryRelationship> <precondition>
<!-- Precondition -->
</precondition></substanceAdministration> |
|
|---|
| Example | | Example | <substanceAdministration classCode="SBADM" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.21.4.7"/> <id root="1.2.3.999" extension="--example only--"/> <code code="DRUG" displayName="Drug therapy" codeSystem="2.16.840.1.113883.5.4"/> <text/> <statusCode code="active"/> <!-- include template 'UV Use Period' (dynamic) .. O -->
<repeatNumber/> <routeCode code="SOAK" displayName="Immersion (soak)" codeSystem="2.16.840.1.113883.5.112"/> <approachSiteCode code="--code--" codeSystem="2.16.840.1.113883.5.1052"/> <administrationUnitCode code="APPFUL" displayName="Applicatorful" codeSystem="2.16.840.1.113883.5.85"/> <subject>
<!-- template 'CDA Subject (Body)' (dynamic) -->
</subject> <consumable typeCode="CSM">
<!-- template 2.16.840.1.113883.10.12.312 'CDA ManufacturedProduct' (dynamic) -->
</consumable> <!-- choice: 1..1
element hl7:author
element hl7:participant[@typeCode='AUT']
-->
<!-- choice: 0..1
element hl7:informant[exists(hl7:assignedEntity)]
element hl7:participant[@typeCode='INF']
element hl7:informant[exists(hl7:relatedEntity)]
-->
<participant typeCode="RCT">
<!-- template 2.16.840.1.113883.10.12.321 'CDA Participant (Body)' (dynamic) -->
</participant> <participant typeCode="VRF">
<!-- template 2.16.840.1.113883.10.12.321 'CDA Participant (Body)' (dynamic) -->
</participant> <entryRelationship typeCode="COMP">
<sequenceNumber value="1"/> <!-- template 2.16.840.1.113883.10.21.4.6 'Subordinate Substance Administration' (dynamic) -->
</entryRelationship> <entryRelationship typeCode="REFR">
<!-- template 2.16.840.1.113883.10.21.4.8 'UV Medication Order Reference' (dynamic) -->
</entryRelationship> <entryRelationship typeCode="REFR">
<!-- template 2.16.840.1.113883.10.21.4.9 'UV Dispense Event Reference' (dynamic) -->
</entryRelationship></substanceAdministration> |
|
|---|
| Example | | Example | <substanceAdministration classCode="SBADM" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.21.4.7"/> <id root="1.2.3.99.99.99" extension="988437489739"/> <code code="..." codeSystem="..."/> <text>...</text> <statusCode code="active"/> <effectiveTime value="..."/> <repeatNumber value="..."/> <routeCode code="SOAK" codeSystem="2.16.840.1.113883.5.112" displayName="Immersion (soak)"/> <approachSiteCode code="..." codeSystem="2.16.840.1.113883.5.1052"/> <administrationUnitCode code="PUFF" displayName="Puff" codeSystem="2.16.840.1.113883.5.85"/> <consumable typeCode="CSM">
<!-- Consumable -->
</consumable> <participant typeCode="DEV">
<!-- Device -->
</participant> <participant typeCode="LOC">
<!-- Location -->
</participant> <entryRelationship typeCode="COMP">
<!-- Subordinate Substance Administrations -->
</entryRelationship> <entryRelationship typeCode="COMP">
<!-- Annotations -->
</entryRelationship> <precondition>
<!-- Precondition -->
</precondition></substanceAdministration> |
|
|---|
| Item | DT | Card | Conf | Description | Label |
|---|
hl7:substanceAdministration
|
| | | | | Medi...ment |  | @classCode
|
| cs | 1 … 1 | F | SBADM |  | @moodCode
|
| cs | 1 … 1 | R | EVN will be used to record a medication statement where the patient is currently taking or has taken the medication in the past.
INT will be used to record a medication statement where the patient plans to take the medication or be administered the medication in the future. | | | CONF | | @moodCode shall be "EVN" | | or | | @moodCode shall be "INT" |
|  | hl7:templateId
|
| II | 1 … 1 | M | | Medi...ment |  |  | @root
|
| uid | 1 … 1 | F | 2.16.840.1.113883.10.21.4.7 |  | hl7:id
|
| II | 0 … * | R | | Medi...ment |  | hl7:code
|
| CD (preferred) | 0 … 1 | R | The code element is valorized with the ACT code DRUG; FD or IMMUNIZ unless it is used for asserting the known absence of medication treatments or no information about them. | Medi...ment | | | CONF | | The value of @code comes preferably from value set 2.16.840.1.113883.1.11.19708 ActSubstanceAdministrationCode (DYNAMIC) | | or | | The value of @code comes preferably from value set 2.16.840.1.113883.11.21.5 Unknown or absent medication (DYNAMIC) |
|  | hl7:text
|
| ED | 0 … 1 | | | Medi...ment |  | hl7:statusCode
|
| CS (required) | 1 … 1 | M | | Medi...ment | | | CONF | | The value of @code shall be drawn from value set 2.16.840.1.113883.1.11.15933 ActStatus (DYNAMIC) |
| | Included | | | from 2.16.840.1.113883.10.21.9.1 UV Use Period (DYNAMIC) | | Choice | 1 … 1 | | The effectiveTime element encodes the use period of the medication, it is always expressed as an interval of time.
It may be expressed using the low and high OR with the width element.
The first is used to indicate a specified interval (e.g. from march 15th, 2017); the latter for indicating a 'floating' period (e.g. 2 weeks). Elements to choose from:- hl7:effectiveTime[hl7:low | hl7:high][not(hl7:width)]
- hl7:effectiveTime[hl7:width][not(hl7:low|hl7:high)]
- hl7:effectiveTime[hl7:low | hl7:width][not(hl7:high)]
|  |  | hl7:effectiveTime
|
| IVL_TS | 0 … 1 | C | Case 1: specified interval
The low and high values of the first effectiveTime element represent the start and stop times for the medication. The low value represents the start time, and the high value represents the stop time. If either the low or the high value is unknown, this shall be recorded by setting the nullFlavor attribute to UNK.
In case of unbounded period (continuous therapy) the high element will be valued with the nullFlavor attribute to NA.
The high value records the end of the medication regime according to the information provided in the prescription or order. For example, if the prescription is for enough medication to last 30 days, then the high value should contain a date that is 30 days later then the low value. The rationale is that a provider, seeing a prescription that has not been refilled would normally assume that the medication is no longer being taken, even if the intent of the treatment plan is to continue the medication indefinitely. | Medi...ment | where [hl7:low or
[not(hl7:width)] | | | cs | 0 … 1 | | | | | Example | Known Interval <effectiveTime type="IVL_TS">
<low value="20130321"/> <high value="20140321"/></effectiveTime> | | | Example | Information not available about the period <effectiveTime type="IVL_TS" nullFlavor="NI"/> | | | Example | Unknown end date <effectiveTime type="IVL_TS">
<low value="20130321"/> <high nullFlavor="UNK"/></effectiveTime> | | | Example | continous therapy <effectiveTime type="IVL_TS">
<low value="20130321"/> <high nullFlavor="NA"/></effectiveTime> | | IVXB_TS | 1 … 1 | R | | Medi...ment | | IVXB_TS | 0 … 1 | R | | Medi...ment |  |  | hl7:effectiveTime
|
| IVL_TS | 0 … 1 | C | Case 2: 'floating' period:
The width element is used to specify a period of (actual or intended) administration that is not anchored to any specific date (e.g. a two weeks therapy) | Medi...ment | where [hl7:width] [not(hl7:lowor
hl7:high)] | | | | Example | 2 week period <effectiveTime type="IVL_TS">
<width value="2" unit="w"/></effectiveTime> | | | | NP | | Medi...ment | | | | NP | | Medi...ment | | | | NP | | Medi...ment | | PQ | 1 … 1 | R | | Medi...ment | | cs | 1 … 1 | R | | | | CONF | | The value of @unit shall be drawn from value set 2.16.840.1.113883.11.21.1 Medication Time Units (UCUM) (DYNAMIC) |
|  |  | hl7:effectiveTime
|
| IVL_TS | 0 … 1 | C | Case 3: anchored period:
The width element is used to specify a period of (actual or intended) administration anchored to a specific date (e.g. a two weeks therapy starting today) | Medi...ment | where [hl7:low or
[not(hl7:high)] | | | | Example | 2 week period starting on 2013-03-21 <effectiveTime type="IVL_TS">
<low value="20130321"/> <width value="2" unit="w"/></effectiveTime> | | IVXB_TS | 0 … 1 | C | | Medi...ment | | PQ | 1 … 1 | R | | Medi...ment | | cs | 1 … 1 | R | | | | CONF | | The value of @unit shall be drawn from value set 2.16.840.1.113883.11.21.1 Medication Time Units (UCUM) (DYNAMIC) |
|  | hl7:repeatNumber
|
| IVL_INT | 0 … 1 | | | Medi...ment |  | hl7:routeCode
|
| CE (example) | 0 … 1 | | | Medi...ment | | | CONF | | Examples of the value of @code are in the value set 2.16.840.1.113883.1.11.14581 RouteOfAdministration (DYNAMIC) |
|  | hl7:approachSiteCode
|
| CD (example) | 0 … * | | | Medi...ment | | | CONF | | Examples of the value of @code are in the value set 2.16.840.1.113883.1.11.19724 HumanSubstanceAdministrationSite (DYNAMIC) |
|  | hl7:doseQuantity
|
| IVL_PQ | | NP | | Medi...ment |  | hl7:rateQuantity
|
| IVL_PQ | | NP | | Medi...ment |  | hl7:maxDoseQuantity
|
| RTO_PQ_PQ | 0 … 1 | | | Medi...ment |  | hl7:administrationUnitCode
|
| CE | | NP | | Medi...ment |  | hl7:subject
|
| | 0 … 1 | C | Patient: The patient that takes the medicine.
Contains 2.16.840.1.113883.10.12.320 CDA Subject (Body) (DYNAMIC) | Medi...ment | | | Constraint | Condition: This can be omitted if the patient context that is provided in the CDA header is identical to the subject | | Choice | | | Elements to choose from:- hl7:consumable containing template 2.16.840.1.113883.10.21.4.10 UV Medication Information (simple) (DYNAMIC)
- hl7:consumable containing template 2.16.840.1.113883.10.21.4.11 UV Medication Information (detail) (DYNAMIC)
|  |  | hl7:consumable
|
| | 0 … 1 | R | Consumable: The medication that is administered (simple)
Contains 2.16.840.1.113883.10.21.4.10 UV Medication Information (simple) (DYNAMIC) | Medi...ment | | cs | 1 … 1 | F | CSM |  |  | hl7:consumable
|
| | 0 … 1 | R | Consumable: The medication that is administered (detail)
Contains 2.16.840.1.113883.10.21.4.11 UV Medication Information (detail) (DYNAMIC) | Medi...ment | | cs | 1 … 1 | F | CSM | | Choice | | | Required author of the medication statement: healthcare professional or patient Elements to choose from:- hl7:author containing template 2.16.840.1.113883.10.12.318 CDA Author (Body) (DYNAMIC)
- hl7:participant[@typeCode='AUT']
|  |  | hl7:author
|
| | | | Use this if the author of the medication statement is a healthcare professional
Contains 2.16.840.1.113883.10.12.318 CDA Author (Body) (DYNAMIC) | Medi...ment | | | Example | Author of the medication statement is a healthcare professional <author>
<time value="20170221"/> <assignedAuthor>
<id root="1.2.3.99.99.99" extension="75487435893498"/> <assignedPerson>
<name>
<given qualifier="IN">Ampu</given> <prefix qualifier="VV">L.</prefix> <family>Lee</family> </name> </assignedPerson> </assignedAuthor></author> |  |  | hl7:participant
|
| | | | Use this if the author of the medication statement is the patient | Medi...ment | | where [@typeCode='AUT'] | | | cs | 1 … 1 | F | AUT | | | Example | Author of the medication statement is the patient <participant typeCode="AUT">
<time value="20170121091548"/> <participantRole classCode="PAT"/></participant> | | TS | 1 … 1 | R | | Medi...ment | | | 1 … 1 | M | | Medi...ment | | cs | 1 … 1 | F | PAT | | Choice | | | Optional informants of the medication statement: healthcare professional or patient contact party (related party) Elements to choose from:- hl7:informant[exists(hl7:assignedEntity)]
- hl7:participant[@typeCode='INF']
- hl7:informant[exists(hl7:relatedEntity)]
|  |  | hl7:informant
|
| | | | Use this if the informant of the medication statement is a healthcare professional | Medi...ment | | where [exists(hl7:assignedEntity)] | | | cs | 0 … 1 | F | INF | | cs | 0 … 1 | F | OP | | | Example | Informant of the medication statement is a healthcare professional <informant>
<assignedEntity>
<id root="1.2.3.99.99.99" extension="75487435893498"/> <assignedPerson>
<name>
<given qualifier="IN">Ampu</given> <prefix qualifier="VV">L.</prefix> <family>Lee</family> </name> </assignedPerson> </assignedEntity></informant> | | | 1 … 1 | | Contains 2.16.840.1.113883.10.12.153 CDA AssignedEntity (DYNAMIC) | Medi...ment |  |  | hl7:participant
|
| | | | Use this if the informant of the medication statement is the patient | Medi...ment | | where [@typeCode='INF'] | | | cs | 1 … 1 | F | INF | | | Example | Informant of the medication statement is the patient <participant typeCode="INF">
<time value="20170121091548"/> <participantRole classCode="PAT"/></participant> | | TS | 1 … 1 | R | | Medi...ment | | | 1 … 1 | M | | Medi...ment | | cs | 1 … 1 | F | PAT |  |  | hl7:informant
|
| | | | Use this if the informant of the medication statement is a contact party (related party) | Medi...ment | | where [exists(hl7:relatedEntity)] | | | cs | 0 … 1 | F | INF | | cs | 0 … 1 | F | OP | | | Example | Informant of the medication statement is a contact party (related party) <informant>
<relatedEntity classCode="AGNT">
<relatedPerson classCode="PSN" determinerCode="INSTANCE">
<name>
<!-- .. -->
</name> </relatedPerson> </relatedEntity></informant> | | | 1 … 1 | | Contains 2.16.840.1.113883.10.12.316 CDA RelatedEntity (DYNAMIC) | Medi...ment |  | hl7:participant
|
| | 0 … 1 | | Record Target: indicates the person who's medical record holds the documentation of this medication statement. This element is only populated when the document is placed in a medical record of someone other than the patient (subject).
Contains 2.16.840.1.113883.10.12.321 CDA Participant (Body) (DYNAMIC) | Medi...ment | | where [@typeCode='RCT'] | |  |  | @typeCode
|
| cs | 1 … 1 | F | RCT |  | hl7:participant
|
| | 0 … 1 | | Verifier: The person or organization that has primary responsibility for the medication statement. The responsible party is not necessarily present in an action, but is accountable for the action through the power to delegate.
Contains 2.16.840.1.113883.10.12.321 CDA Participant (Body) (DYNAMIC) | Medi...ment | | where [@typeCode='VRF'] | |  |  | @typeCode
|
| cs | 1 … 1 | F | VRF |  | hl7:entryRelationship
|
| | 0 … * | C | Subordinate Substance Administration Statament as a component of the overall medication statement.
At least one subordinated <substanceAdministration> has to be present to convey information about dosages (dose, frequency of intakes,..) unless medications are unknown or known absent.
Subordinated <substanceAdministration> elements can be also used either to handle split dosing, or to support combination medications.
Contains 2.16.840.1.113883.10.21.4.6 UV Subordinate Substance Administration (DYNAMIC) | Medi...ment | | where [exists(hl7:substanceAdministration)] | |  |  | @typeCode
|
| cs | 1 … 1 | F | COMP | | | Constraint | At least one subordinate <substanceAdministration> element SHALL be present unless medications are unknown or known absent.</substanceAdministration>
| | | Example | <entryRelationship typeCode="COMP">
<!-- component: Subordinate Substance Administration Statement. -->
<substanceAdministration classCode="SBADM" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.21.4.6"/> <!-- .. -->
</substanceAdministration></entryRelationship> |  |  | hl7:sequenceNumber
|
| INT | 0 … 1 | | Sequence number of the Subordinate Substance Administration. | Medi...ment |  | hl7:entryRelationship
|
| | 0 … * | R | Medication Order Reference.
Contains 2.16.840.1.113883.10.21.4.8 UV Medication Order Reference (DYNAMIC) | Medi...ment | | where [@typeCode='REFR' and exists(hl7:substanceAdministration)] | |  |  | @typeCode
|
| cs | 1 … 1 | F | REFR | | | Example | <entryRelationship typeCode="REFR">
<substanceAdministration classCode="SBADM" moodCode="RQO">
<templateId root="2.16.840.1.113883.10.21.4.8"/> <!-- .. -->
</substanceAdministration></entryRelationship> |  | hl7:entryRelationship
|
| | 0 … * | R | Dispense Event Reference.
Contains 2.16.840.1.113883.10.21.4.9 UV Dispense Event Reference (DYNAMIC) | Medi...ment | | where [@typeCode='REFR' and exists(hl7:supply)] | |  |  | @typeCode
|
| cs | 1 … 1 | F | REFR | | | Example | <entryRelationship typeCode="REFR">
<supply classCode="SPLY" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.21.4.9"/> <!-- .. -->
</supply></entryRelationship> | | Choice | | | Elements to choose from:- hl7:entryRelationship[@typeCode='RSON' and exists(hl7:observation)] containing template 2.16.840.1.113883.10.21.4.3 UV ClinicalStatement Observation (DYNAMIC)
- hl7:entryRelationship[@typeCode='RSON' and exists(hl7:act)] containing template 2.16.840.1.113883.10.22.4.31 IPS Internal Reference (DYNAMIC)
|  |  | hl7:entryRelationship
|
| | 0 … * | R | Reason: Specifies the reason (indication) for authoring the order.
Contains 2.16.840.1.113883.10.21.4.3 UV ClinicalStatement Observation (DYNAMIC) | Medi...ment | | where [@typeCode='RSON' and exists(hl7:observation)] | | | cs | 1 … 1 | F | RSON | | | Example | <cda:entryRelationship typeCode="RSON">
<priorityNumber value="1"/> <!-- template 2.16.840.1.113883.10.21.4.3 'UV ClinicalStatement Observation' (2016-05-01T00:00:00) -->
</cda:entryRelationship> | | INT.NONNEG | 0 … 1 | R | Indicates the priority of this reason for the order in relation to its sibling reasons. | Medi...ment |  |  | hl7:entryRelationship
|
| | 0 … * | R | Reason: Specifies the reason (indication) for authoring the order.
Contains 2.16.840.1.113883.10.22.4.31 IPS Internal Reference (DYNAMIC) | Medi...ment | | where [@typeCode='RSON' and exists(hl7:act)] | | | cs | 1 … 1 | F | RSON | | | Example | <entryRelationship typeCode="RSON">
<priorityNumber value="1"/> <act>
<!-- Clinical Statement Minimal -->
</act></entryRelationship> | | INT.NONNEG | 0 … 1 | R | Indicates the priority of this reason for the order in relation to its sibling reasons. | Medi...ment |
|
UV Medication Administration
The following graph gives an overview of the high-level template components of this template, followed by the actual definition.
-
Entry UV Medication Administration (2.16.840.1.113883.10.21.4.13)
-
Entry UV Use Period (2.16.840.1.113883.10.21.9.1)
-
Entry CDA Subject (Body) (2.16.840.1.113883.10.12.320)
-
Entry UV Medication Information (simple) (2.16.840.1.113883.10.21.4.10)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry UV Medication Information (detail) (2.16.840.1.113883.10.21.4.11)
-
Entry UV Content (2.16.840.1.113883.10.21.4.17)
-
Entry UV Generalized Medicine Class (2.16.840.1.113883.10.21.4.19)
-
Entry UV Content (2.16.840.1.113883.10.21.4.17)
-
Entry UV Generalized Medicine Class (2.16.840.1.113883.10.21.4.19)
-
Entry UV Content (2.16.840.1.113883.10.21.4.17)
-
Entry UV Generalized Medicine Class (2.16.840.1.113883.10.21.4.19)
-
Entry UV Content (2.16.840.1.113883.10.21.4.17)
-
Entry UV Generalized Medicine Class (2.16.840.1.113883.10.21.4.19)
-
Entry UV Ingredient (2.16.840.1.113883.10.21.4.18)
-
Entry UV Ingredient (2.16.840.1.113883.10.21.4.18)
-
Entry UV Ingredient (2.16.840.1.113883.10.21.4.18)
-
Entry UV Ingredient (2.16.840.1.113883.10.21.4.18)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA Author (Body) (2.16.840.1.113883.10.12.318)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
* CDA AssignedEntity (2.16.840.1.113883.10.12.153)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA RelatedEntity (2.16.840.1.113883.10.12.316)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
Entry CDA Participant (Body) (2.16.840.1.113883.10.12.321)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
Entry CDA PlayingEntity (2.16.840.1.113883.10.12.313)
-
Entry CDA Participant (Body) (2.16.840.1.113883.10.12.321)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
Entry CDA PlayingEntity (2.16.840.1.113883.10.12.313)
-
Entry CDA Participant (Body) (2.16.840.1.113883.10.12.321)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
Entry CDA PlayingEntity (2.16.840.1.113883.10.12.313)
-
Entry UV Subordinate Substance Administration (2.16.840.1.113883.10.21.4.6)
-
Entry UV Substitution Event Adminstration (2.16.840.1.113883.10.21.4.14)
-
Entry UV Medication Order Reference (2.16.840.1.113883.10.21.4.8)
-
Entry CDA Author (Body) (2.16.840.1.113883.10.12.318)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry UV Dispense Event Reference (2.16.840.1.113883.10.21.4.9)
-
Entry UV ClinicalStatement Observation (2.16.840.1.113883.10.21.4.3)
-
Entry CDA Subject (Body) (2.16.840.1.113883.10.12.320)
-
Entry CDA Specimen (2.16.840.1.113883.10.12.322)
-
Entry CDA Performer (Body) (2.16.840.1.113883.10.12.323)
-
* CDA AssignedEntity (2.16.840.1.113883.10.12.153)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA Author (Body) (2.16.840.1.113883.10.12.318)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA Informant (Body) (2.16.840.1.113883.10.12.319)
-
* CDA AssignedEntity (2.16.840.1.113883.10.12.153)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA RelatedEntity (2.16.840.1.113883.10.12.316)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
Entry CDA Participant (Body) (2.16.840.1.113883.10.12.321)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
Entry CDA PlayingEntity (2.16.840.1.113883.10.12.313)
-
Entry CDA Reference (2.16.840.1.113883.10.12.324)
-
Entry CDA ExternalAct (2.16.840.1.113883.10.12.325)
-
Entry CDA ExternalObservation (2.16.840.1.113883.10.12.326)
-
Entry CDA ExternalProcedure (2.16.840.1.113883.10.12.327)
-
Entry CDA ExternalDocument (2.16.840.1.113883.10.12.328)
-
Entry CDA Precondition (2.16.840.1.113883.10.12.329)
-
Entry IPS Internal Reference (2.16.840.1.113883.10.22.4.31)
|
The boxes reflect the CDA Template Types. Symbols: * denotes templates with more than one classification, @ indicates a recursion in the definition
| Id | 2.16.840.1.113883.10.21.4.13 | Effective Date | 2023‑01‑30 08:31:37Other versions this id:  UVMedicationadministration as of 2019‑02‑17 UVMedicationadministration as of 2019‑02‑17
|
|---|
| Status |  Draft Draft | Version Label | 2023 |
|---|
| Name | UVMedicationadministration | Display Name | UV Medication Administration |
|---|
| Description | Universal Medication Administration: This includes information about an actual administration of a medication. Medication administrations include information about medication use where the medications have been prescribed or not prescribed. Medication administrations may include "negative" statements such as "the patient was not given medication ABC". Due to
implementation experience, dosage information is always put into the subordinate substance administration entries. |
|
| Context | Parent nodes of template element with id 2.16.840.1.113883.10.21.4.13 |
|---|
| Label | MedicationAdministration
|
|---|
| Classification | CDA Entry Level Template |
|---|
| Open/Closed | Open (other than defined elements are allowed) |
|---|
| Uses | | Uses 14 templates | | Uses | as | Name | Version |
|---|
| 2.16.840.1.113883.10.21.9.1 | Include |  UV Use Period (2023) UV Use Period (2023) | DYNAMIC | | 2.16.840.1.113883.10.12.320 | Containment |  CDA Subject (Body) CDA Subject (Body) | DYNAMIC | | 2.16.840.1.113883.10.21.4.10 | Containment |  UV Medication Information (simple) (R1-STU2-ballot) UV Medication Information (simple) (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.21.4.11 | Containment |  UV Medication Information (detail) (2023) UV Medication Information (detail) (2023) | DYNAMIC | | 2.16.840.1.113883.10.12.318 | Containment |  CDA Author (Body) CDA Author (Body) | DYNAMIC | | 2.16.840.1.113883.10.12.153 | Containment |  CDA AssignedEntity CDA AssignedEntity | DYNAMIC | | 2.16.840.1.113883.10.12.316 | Containment |  CDA RelatedEntity CDA RelatedEntity | DYNAMIC | | 2.16.840.1.113883.10.12.321 | Containment |  CDA Participant (Body) CDA Participant (Body) | DYNAMIC | | 2.16.840.1.113883.10.21.4.6 | Containment |  UV Subordinate Substance Administration (2023) UV Subordinate Substance Administration (2023) | DYNAMIC | | 2.16.840.1.113883.10.21.4.14 | Containment |  UV Substitution Event Adminstration (2023) UV Substitution Event Adminstration (2023) | DYNAMIC | | 2.16.840.1.113883.10.21.4.8 | Containment |  UV Medication Order Reference (R1-STU2-ballot) UV Medication Order Reference (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.21.4.9 | Containment |  UV Dispense Event Reference (R1-STU2-ballot) UV Dispense Event Reference (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.21.4.3 | Containment |  UV ClinicalStatement Observation (R1-STU2-ballot) UV ClinicalStatement Observation (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.22.4.31 | Containment |  IPS Internal Reference (STU1) IPS Internal Reference (STU1) | DYNAMIC |
|
|
|---|
| Relationship | Version: template 2.16.840.1.113883.10.21.4.13 UV Medication Administration (2019‑02‑17)
Specialization: template 2.16.840.1.113883.10.12.308 CDA SubstanceAdministration (2005‑09‑07) ref ad1bbr- |
|---|
| Example | | Example | <substanceAdministration classCode="SBADM" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.21.4.13"/> <id root="1.2.3.99.99.99" extension="988437489739"/> <code code="DRUG" codeSystem="2.16.840.1.113883.5.4"/> <text>...</text> <statusCode code="active"/> <effectiveTime value="..."/> <repeatNumber value="..."/> <routeCode code="SOAK" codeSystem="2.16.840.1.113883.5.112" displayName="Immersion (soak)"/> <approachSiteCode code="..." codeSystem="2.16.840.1.113883.5.1052"/> <administrationUnitCode code="PUFF" displayName="Puff" codeSystem="2.16.840.1.113883.5.85"/> <consumable typeCode="CSM">
<!-- Consumable -->
</consumable> <participant typeCode="DEV">
<!-- Device -->
</participant> <participant typeCode="LOC">
<!-- Location -->
</participant> <entryRelationship typeCode="COMP">
<!-- Subordinate Substance Administrations -->
</entryRelationship> <entryRelationship typeCode="COMP">
<!-- Annotations -->
</entryRelationship> <precondition>
<!-- Precondition -->
</precondition></substanceAdministration> |
|
|---|
| Example | | Example | <substanceAdministration classCode="SBADM" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.21.4.13"/> <id root="1.2.3.999" extension="--example only--"/> <code code="DRUG" codeSystem="2.16.840.1.113883.5.4"/> <text>...</text> <statusCode code="active"/> <!-- include template 'UV Use Period' (dynamic) .. O -->
<routeCode code="SOAK" displayName="Immersion (soak)" codeSystem="2.16.840.1.113883.5.112"/> <approachSiteCode code="--code--" codeSystem="2.16.840.1.113883.5.1052"/> <administrationUnitCode code="APPFUL" displayName="Applicatorful" codeSystem="2.16.840.1.113883.5.85"/> <subject>
<!-- template 'CDA Subject (Body)' (dynamic) -->
</subject> <consumable typeCode="CSM">
<!-- template 2.16.840.1.113883.10.12.312 'CDA ManufacturedProduct' (dynamic) -->
</consumable> <!-- choice: 1..1
element hl7:author
element hl7:participant[@typeCode='AUT']
-->
<participant typeCode="RCT">
<!-- template 2.16.840.1.113883.10.12.321 'CDA Participant (Body)' (dynamic) -->
</participant> <participant typeCode="VRF">
<!-- template 2.16.840.1.113883.10.12.321 'CDA Participant (Body)' (dynamic) -->
</participant> <entryRelationship typeCode="COMP">
<sequenceNumber value="1"/> <!-- template 2.16.840.1.113883.10.21.4.6 'Subordinate Substance Administration' (dynamic) -->
</entryRelationship> <entryRelationship typeCode="REFR">
<!-- template 2.16.840.1.113883.10.21.4.8 'UV Medication Order Reference' (dynamic) -->
</entryRelationship> <entryRelationship typeCode="REFR">
<!-- template 2.16.840.1.113883.10.21.4.9 'UV Dispense Event Reference' (dynamic) -->
</entryRelationship></substanceAdministration> |
|
|---|
| Item | DT | Card | Conf | Description | Label |
|---|
hl7:substanceAdministration
|
| | | | | Medi...tion |  | @classCode
|
| cs | 1 … 1 | F | SBADM |  | @moodCode
|
| cs | 1 … 1 | F | EVN |  | hl7:templateId
|
| II | 1 … 1 | M | | Medi...tion |  |  | @root
|
| uid | 1 … 1 | F | 2.16.840.1.113883.10.21.4.13 |  | hl7:id
|
| II | 0 … * | R | | Medi...tion | | | Constraint | If the use case requires updates on the order, the ID shall be made mandatory. |  | hl7:code
|
| CD (extensible) | 0 … 1 | | The code element is valorized with the ACT code from the indicated value set unless it is used for asserting the known absence of medication treatments or no information about them. | Medi...tion | | | CONF | | The value of @code should be drawn from value set 2.16.840.1.113883.1.11.19708 ActSubstanceAdministrationCode (DYNAMIC) | | or | | The value of @code should be drawn from value set 2.16.840.1.113883.11.21.5 Unknown or absent medication (DYNAMIC) |
|  | hl7:text
|
| ED | 0 … 1 | | | Medi...tion |  | hl7:statusCode
|
| CS (required) | 1 … 1 | M | | Medi...tion | | | CONF | | The value of @code shall be drawn from value set 2.16.840.1.113883.1.11.15933 ActStatus (DYNAMIC) |
| | Included | | | from 2.16.840.1.113883.10.21.9.1 UV Use Period (DYNAMIC) | | Choice | 1 … 1 | | The effectiveTime element encodes the use period of the medication, it is always expressed as an interval of time.
It may be expressed using the low and high OR with the width element.
The first is used to indicate a specified interval (e.g. from march 15th, 2017); the latter for indicating a 'floating' period (e.g. 2 weeks). Elements to choose from:- hl7:effectiveTime[hl7:low | hl7:high][not(hl7:width)]
- hl7:effectiveTime[hl7:width][not(hl7:low|hl7:high)]
- hl7:effectiveTime[hl7:low | hl7:width][not(hl7:high)]
|  |  | hl7:effectiveTime
|
| IVL_TS | 0 … 1 | C | Case 1: specified interval
The low and high values of the first effectiveTime element represent the start and stop times for the medication. The low value represents the start time, and the high value represents the stop time. If either the low or the high value is unknown, this shall be recorded by setting the nullFlavor attribute to UNK.
In case of unbounded period (continuous therapy) the high element will be valued with the nullFlavor attribute to NA.
The high value records the end of the medication regime according to the information provided in the prescription or order. For example, if the prescription is for enough medication to last 30 days, then the high value should contain a date that is 30 days later then the low value. The rationale is that a provider, seeing a prescription that has not been refilled would normally assume that the medication is no longer being taken, even if the intent of the treatment plan is to continue the medication indefinitely. | Medi...tion | where [hl7:low or
[not(hl7:width)] | | | cs | 0 … 1 | | | | | Example | Known Interval <effectiveTime type="IVL_TS">
<low value="20130321"/> <high value="20140321"/></effectiveTime> | | | Example | Information not available about the period <effectiveTime type="IVL_TS" nullFlavor="NI"/> | | | Example | Unknown end date <effectiveTime type="IVL_TS">
<low value="20130321"/> <high nullFlavor="UNK"/></effectiveTime> | | | Example | continous therapy <effectiveTime type="IVL_TS">
<low value="20130321"/> <high nullFlavor="NA"/></effectiveTime> | | IVXB_TS | 1 … 1 | R | | Medi...tion | | IVXB_TS | 0 … 1 | R | | Medi...tion |  |  | hl7:effectiveTime
|
| IVL_TS | 0 … 1 | C | Case 2: 'floating' period:
The width element is used to specify a period of (actual or intended) administration that is not anchored to any specific date (e.g. a two weeks therapy) | Medi...tion | where [hl7:width] [not(hl7:lowor
hl7:high)] | | | | Example | 2 week period <effectiveTime type="IVL_TS">
<width value="2" unit="w"/></effectiveTime> | | | | NP | | Medi...tion | | | | NP | | Medi...tion | | | | NP | | Medi...tion | | PQ | 1 … 1 | R | | Medi...tion | | cs | 1 … 1 | R | | | | CONF | | The value of @unit shall be drawn from value set 2.16.840.1.113883.11.21.1 Medication Time Units (UCUM) (DYNAMIC) |
|  |  | hl7:effectiveTime
|
| IVL_TS | 0 … 1 | C | Case 3: anchored period:
The width element is used to specify a period of (actual or intended) administration anchored to a specific date (e.g. a two weeks therapy starting today) | Medi...tion | where [hl7:low or
[not(hl7:high)] | | | | Example | 2 week period starting on 2013-03-21 <effectiveTime type="IVL_TS">
<low value="20130321"/> <width value="2" unit="w"/></effectiveTime> | | IVXB_TS | 0 … 1 | C | | Medi...tion | | PQ | 1 … 1 | R | | Medi...tion | | cs | 1 … 1 | R | | | | CONF | | The value of @unit shall be drawn from value set 2.16.840.1.113883.11.21.1 Medication Time Units (UCUM) (DYNAMIC) |
|  | hl7:repeatNumber
|
| IVL_INT | 0 … 1 | | | Medi...tion |  | hl7:routeCode
|
| CE (example) | 0 … 1 | | | Medi...tion | | | CONF | | Examples of the value of @code are in the value set 2.16.840.1.113883.1.11.14581 RouteOfAdministration (DYNAMIC) |
|  | hl7:approachSiteCode
|
| CD (example) | 0 … * | | | Medi...tion | | | CONF | | Examples of the value of @code are in the value set 2.16.840.1.113883.1.11.19724 HumanSubstanceAdministrationSite (DYNAMIC) |
|  | hl7:doseQuantity
|
| IVL_PQ | | NP | | Medi...tion |  | hl7:rateQuantity
|
| IVL_PQ | | NP | | Medi...tion |  | hl7:maxDoseQuantity
|
| RTO_PQ_PQ | 0 … 1 | | | Medi...tion |  | hl7:administrationUnitCode
|
| CE | | NP | | Medi...tion |  | hl7:subject
|
| | 0 … 1 | C | Patient: The patient that takes the medicine.
Contains 2.16.840.1.113883.10.12.320 CDA Subject (Body) (DYNAMIC) | Medi...tion | | | Constraint | Condition: This can be omitted if the patient context that is provided in the CDA header is identical to the subject | | Choice | 1 … 1 | | Elements to choose from:- hl7:consumable containing template 2.16.840.1.113883.10.21.4.10 UV Medication Information (simple) (DYNAMIC)
- hl7:consumable containing template 2.16.840.1.113883.10.21.4.11 UV Medication Information (detail) (DYNAMIC)
|  |  | hl7:consumable
|
| | 0 … 1 | R | Consumable: The medication that is administered (simple)
Contains 2.16.840.1.113883.10.21.4.10 UV Medication Information (simple) (DYNAMIC) | Medi...tion | | cs | 1 … 1 | F | CSM |  |  | hl7:consumable
|
| | 0 … 1 | R | Consumable: The medication that is administered (detail)
Contains 2.16.840.1.113883.10.21.4.11 UV Medication Information (detail) (DYNAMIC) | Medi...tion | | cs | 1 … 1 | F | CSM | | Choice | 1 … 1 | | Required author of the medication administration: healthcare professional or patient Elements to choose from:- hl7:author containing template 2.16.840.1.113883.10.12.318 CDA Author (Body) (DYNAMIC)
- hl7:participant[@typeCode='AUT']
|  |  | hl7:author
|
| | | | Use this if the author of the medication statement is a healthcare professional
Contains 2.16.840.1.113883.10.12.318 CDA Author (Body) (DYNAMIC) | Medi...tion | | | Example | Author of the medication statement is a healthcare professional <author>
<time value="20170221"/> <assignedAuthor>
<id root="1.2.3.99.99.99" extension="75487435893498"/> <assignedPerson>
<name>
<given qualifier="IN">Ampu</given> <prefix qualifier="VV">L.</prefix> <family>Lee</family> </name> </assignedPerson> </assignedAuthor></author> |  |  | hl7:participant
|
| | | | Use this if the author of the medication administration is the patient | Medi...tion | | where [@typeCode='AUT'] | | | cs | 1 … 1 | F | AUT | | | Example | Author of the medication statement is the patient <participant typeCode="AUT">
<time value="20170121091548"/> <participantRole classCode="PAT"/></participant> | | TS | 1 … 1 | R | | Medi...tion | | | 1 … 1 | M | | Medi...tion | | cs | 1 … 1 | F | PAT | | Choice | 0 … 1 | | Optional informants of the medication administration: healthcare professional or patient contact party (related party) Elements to choose from:- hl7:informant[exists(hl7:assignedEntity)]
- hl7:participant[@typeCode='INF']
- hl7:informant[exists(hl7:relatedEntity)]
|  |  | hl7:informant
|
| | | | Use this if the informant of the medication statement is a healthcare professional | Medi...tion | | where [exists(hl7:assignedEntity)] | | | cs | 0 … 1 | F | INF | | cs | 0 … 1 | F | OP | | | Example | Informant of the medication statement is a healthcare professional <informant>
<assignedEntity>
<id root="1.2.3.99.99.99" extension="75487435893498"/> <assignedPerson>
<name>
<given qualifier="IN">Ampu</given> <prefix qualifier="VV">L.</prefix> <family>Lee</family> </name> </assignedPerson> </assignedEntity></informant> | | | 1 … 1 | | Contains 2.16.840.1.113883.10.12.153 CDA AssignedEntity (DYNAMIC) | Medi...tion |  |  | hl7:participant
|
| | | | Use this if the informant of the medication statement is the patient | Medi...tion | | where [@typeCode='INF'] | | | cs | 1 … 1 | F | INF | | | Example | Informant of the medication statement is the patient <participant typeCode="INF">
<time value="20170121091548"/> <participantRole classCode="PAT"/></participant> | | TS | 1 … 1 | R | | Medi...tion | | | 1 … 1 | M | | Medi...tion | | cs | 1 … 1 | F | PAT |  |  | hl7:informant
|
| | | | Use this if the informant of the medication statement is a contact party (related party) | Medi...tion | | where [exists(hl7:relatedEntity)] | | | cs | 0 … 1 | F | INF | | cs | 0 … 1 | F | OP | | | Example | Informant of the medication statement is a contact party (related party) <informant>
<relatedEntity classCode="AGNT">
<relatedPerson classCode="PSN" determinerCode="INSTANCE">
<name>
<!-- .. -->
</name> </relatedPerson> </relatedEntity></informant> | | | 1 … 1 | | Contains 2.16.840.1.113883.10.12.316 CDA RelatedEntity (DYNAMIC) | Medi...tion |  | hl7:participant
|
| | 0 … 1 | | Record Target: indicates the person who's medical record holds the documentation of this medication statement. This element is only populated when the document is placed in a medical record of someone other than the patient (subject).
Contains 2.16.840.1.113883.10.12.321 CDA Participant (Body) (DYNAMIC) | Medi...tion | | where [@typeCode='RCT'] | |  |  | @typeCode
|
| cs | 1 … 1 | F | RCT |  | hl7:participant
|
| | 0 … 1 | | Verifier: The person or organization that has primary responsibility for the medication statement. The responsible party is not necessarily present in an action, but is accountable for the action through the power to delegate.
Contains 2.16.840.1.113883.10.12.321 CDA Participant (Body) (DYNAMIC) | Medi...tion | | where [@typeCode='VRF'] | |  |  | @typeCode
|
| cs | 1 … 1 | F | VRF |  | hl7:participant
|
| | 0 … 1 | | Location
Contains 2.16.840.1.113883.10.12.321 CDA Participant (Body) (DYNAMIC) | Medi...tion | | where [@typeCode='LOC'] | |  |  | @typeCode
|
| cs | 1 … 1 | F | LOC |  | hl7:entryRelationship
|
| | 0 … * | C | Subordinate Substance Administration Statament as a component of the overall medication statement.
At least one subordinated <substanceAdministration> has to be present to convey information about dosages (dose, frequency of intakes,..) unless medications are unknown or known absent.
Subordinated <substanceAdministration> elements can be also used either to handle split dosing, or to support combination medications.
Contains 2.16.840.1.113883.10.21.4.6 UV Subordinate Substance Administration (DYNAMIC) | Medi...tion | | where [hl7:substanceAdministration] | |  |  | @typeCode
|
| cs | 1 … 1 | F | COMP | | | Constraint | At least one subordinate <substanceAdministration> element SHALL be present unless medications are unknown or known absent.</substanceAdministration>
| | | Example | <entryRelationship typeCode="COMP">
<!-- component: Subordinate Substance Administration Statement. -->
<substanceAdministration classCode="SBADM" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.21.4.6"/> <!-- .. -->
</substanceAdministration></entryRelationship> |  |  | hl7:sequenceNumber
|
| INT | 0 … 1 | | Sequence number of the Subordinate Substance Administration. | Medi...tion |  | hl7:entryRelationship
|
| | 0 … 1 | R | Information about any substitutions in medication that have been made.
Contains 2.16.840.1.113883.10.21.4.14 UV Substitution Event Adminstration (DYNAMIC) | Medi...tion | | where [@typeCode='COMP'] | |  |  | @typeCode
|
| cs | 1 … 1 | F | COMP | | | Example | <entryRelationship typeCode="COMP">
<act classCode="ACT" moodCode="EVN">
<!-- .. -->
</act></entryRelationship> |  | hl7:entryRelationship
|
| | 0 … * | R | Medication Order Reference.
Contains 2.16.840.1.113883.10.21.4.8 UV Medication Order Reference (DYNAMIC) | Medi...tion | | where [@typeCode='REFR'] [hl7:substanceAdministration] | |  |  | @typeCode
|
| cs | 1 … 1 | F | REFR | | | Example | <entryRelationship typeCode="REFR">
<substanceAdministration classCode="SBADM" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.21.4.8"/> <!-- .. -->
</substanceAdministration></entryRelationship> |  | hl7:entryRelationship
|
| | 0 … * | R | Dispense Event Reference.
Contains 2.16.840.1.113883.10.21.4.9 UV Dispense Event Reference (DYNAMIC) | Medi...tion | | where [@typeCode='REFR'] [ [hl7:supply] | |  |  | @typeCode
|
| cs | 1 … 1 | F | REFR | | | Example | <entryRelationship typeCode="REFR">
<supply classCode="SPLY" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.21.4.9"/> <!-- .. -->
</supply></entryRelationship> | | Choice | 0 … * | | Elements to choose from:- hl7:entryRelationship containing template 2.16.840.1.113883.10.21.4.3 UV ClinicalStatement Observation (DYNAMIC)
- hl7:entryRelationship containing template 2.16.840.1.113883.10.22.4.31 IPS Internal Reference (DYNAMIC)
|  |  | hl7:entryRelationship
|
| | 0 … * | R | Reason: Specifies the reason (indication) for authoring the order.
Contains 2.16.840.1.113883.10.21.4.3 UV ClinicalStatement Observation (DYNAMIC) | Medi...tion | | cs | 1 … 1 | F | RSON | | | Example | <cda:entryRelationship typeCode="RSON">
<priorityNumber value="1"/> <!-- template 2.16.840.1.113883.10.21.4.3 'UV ClinicalStatement Observation' (2016-05-01T00:00:00) -->
</cda:entryRelationship> | | INT.NONNEG | 0 … 1 | R | Indicates the priority of this reason for the order in relation to its sibling reasons. | Medi...tion |  |  | hl7:entryRelationship
|
| | 0 … * | R | Reason: Specifies the reason (indication) for authoring the order.
Contains 2.16.840.1.113883.10.22.4.31 IPS Internal Reference (DYNAMIC) | Medi...tion | | cs | 1 … 1 | F | RSON | | | Example | <entryRelationship typeCode="RSON">
<priorityNumber value="1"/> <act>
<!-- Clinical Statement Minimal -->
</act></entryRelationship> | | INT.NONNEG | 0 … 1 | R | Indicates the priority of this reason for the order in relation to its sibling reasons. | Medi...tion |
|
UV Medication Dispense
The following graph gives an overview of the high-level template components of this template, followed by the actual definition.
-
Entry UV Medication Dispense (2.16.840.1.113883.10.21.4.15)
-
Entry CDA Subject (Body) (2.16.840.1.113883.10.12.320)
-
Entry UV Medication Information (simple) (2.16.840.1.113883.10.21.4.10)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry UV Medication Information (detail) (2.16.840.1.113883.10.21.4.11)
-
Entry UV Content (2.16.840.1.113883.10.21.4.17)
-
Entry UV Generalized Medicine Class (2.16.840.1.113883.10.21.4.19)
-
Entry UV Content (2.16.840.1.113883.10.21.4.17)
-
Entry UV Generalized Medicine Class (2.16.840.1.113883.10.21.4.19)
-
Entry UV Content (2.16.840.1.113883.10.21.4.17)
-
Entry UV Generalized Medicine Class (2.16.840.1.113883.10.21.4.19)
-
Entry UV Content (2.16.840.1.113883.10.21.4.17)
-
Entry UV Generalized Medicine Class (2.16.840.1.113883.10.21.4.19)
-
Entry UV Ingredient (2.16.840.1.113883.10.21.4.18)
-
Entry UV Ingredient (2.16.840.1.113883.10.21.4.18)
-
Entry UV Ingredient (2.16.840.1.113883.10.21.4.18)
-
Entry UV Ingredient (2.16.840.1.113883.10.21.4.18)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA Performer (Body) (2.16.840.1.113883.10.12.323)
-
* CDA AssignedEntity (2.16.840.1.113883.10.12.153)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry CDA Participant (Body) (2.16.840.1.113883.10.12.321)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
Entry CDA PlayingEntity (2.16.840.1.113883.10.12.313)
-
Entry CDA Participant (Body) (2.16.840.1.113883.10.12.321)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
Entry CDA PlayingEntity (2.16.840.1.113883.10.12.313)
-
Entry CDA Participant (Body) (2.16.840.1.113883.10.12.321)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
Entry CDA PlayingEntity (2.16.840.1.113883.10.12.313)
-
Entry CDA Participant (Body) (2.16.840.1.113883.10.12.321)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
Entry CDA PlayingEntity (2.16.840.1.113883.10.12.313)
-
Entry UV Medication Order Reference (2.16.840.1.113883.10.21.4.8)
-
Entry CDA Author (Body) (2.16.840.1.113883.10.12.318)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
-
Entry UV Comment Activity (2.16.840.1.113883.10.21.4.12)
-
Entry CDA Author (Body) (2.16.840.1.113883.10.12.318)
-
* CDA Person (2.16.840.1.113883.10.12.152)
-
Entry CDA Device (2.16.840.1.113883.10.12.315)
-
* CDA Organization (2.16.840.1.113883.10.12.151)
|
The boxes reflect the CDA Template Types. Symbols: * denotes templates with more than one classification, @ indicates a recursion in the definition
Entry Level Templates
These entry level templates are used Section "Use Case Entry Level Templates".
UV ClinicalStatement Encounter
UV ClinicalStatement Observation
UV Content
| Id | 2.16.840.1.113883.10.21.4.17 | Effective Date | 2022‑03‑18 08:28:51 |
|---|
| Status |  Under pre-publication review Under pre-publication review | Version Label | R1-STU2-ballot |
|---|
| Name | UVasContent | Display Name | UV Content |
|---|
| Classification | CDA Entry Level Template |
|---|
| Open/Closed | Open (other than defined elements are allowed) |
|---|
|
UV Dispense Request
UV Dispense Event Reference
UV Generalized Medicine Class
UV Ingredient
| Id | 2.16.840.1.113883.10.21.4.18 | Effective Date | 2022‑03‑18 08:34:40 |
|---|
| Status |  Under pre-publication review Under pre-publication review | Version Label | R1-STU2-ballot |
|---|
| Name | UVIngredient | Display Name | UV Ingredient |
|---|
| Classification | CDA Entry Level Template |
|---|
| Open/Closed | Open (other than defined elements are allowed) |
|---|
| Item | DT | Card | Conf | Description | Label |
|---|
| RTO_PQ_PQ | 0 … 1 | | | (UVI...ent) | | | Example | 10 mg of the ingredient per ml <pharm:quantity>
<cda:numerator xsi:type="PQ" value="10" unit="mg"/> <cda:denominator xsi:type="PQ" value="1" unit="ml"/></pharm:quantity> | | | Example | 2% of the ingredient <pharm:quantity>
<cda:numerator xsi:type="PQ" value="2" unit="%"/> <cda:denominator xsi:type="PQ" value="1"/></pharm:quantity> | | | Example | 5mg of the ingredient <pharm:quantity>
<cda:numerator xsi:type="PQ" value="5" unit="mg"/> <cda:denominator xsi:type="PQ" value="1"/></pharm:quantity> |  | cda:numerator
|
| PQ | 0 … 1 | | | (UVI...ent) |  | cda:denominator
|
| PQ | 0 … 1 | | | (UVI...ent) | pharm:ingredientSubstance
|
| | 0 … 1 | | The substance used for this product plying the role indicated in the ingredient classCode.
The <code> element contains the coded representation of the ingredient and the <name> element may be used for the plain text representation.
| (UVI...ent) |  | @classCode
|
| cs | 1 … 1 | F | MMAT |  | @determinerCode
|
| cs | 1 … 1 | F | KIND |  | pharm:code
|
| CD | 0 … 1 | C | | (UVI...ent) |  | pharm:name
|
| EN | 0 … 1 | C | | (UVI...ent) | | | Schematron assert | role | error | | | | test | pharm:code or pharm:name | | | | Message | Either the name or the code of the substance (or both) shall be provided | |
|
UV Medication Information (detail)
| Id | 2.16.840.1.113883.10.21.4.11 | Effective Date | 2023‑02‑03 13:03:38Other versions this id:  UVMedicationInformationdetail as of 2021‑08‑04 12:39:04 UVMedicationInformationdetail as of 2021‑08‑04 12:39:04 UVMedicationInformationdetail as of 2017‑05‑10 UVMedicationInformationdetail as of 2017‑05‑10
|
|---|
| Status |  Draft Draft | Version Label | 2023 |
|---|
| Name | UVMedicationInformationdetail | Display Name | UV Medication Information (detail) |
|---|
| Description | Universal Medication Information (detail) |
|---|
| Context | Parent nodes of template element with id 2.16.840.1.113883.10.21.4.11 |
|---|
| Classification | CDA Entry Level Template |
|---|
| Open/Closed | Open (other than defined elements are allowed) |
|---|
| Uses | | Uses 4 templates | | Uses | as | Name | Version |
|---|
| 2.16.840.1.113883.10.21.4.17 | Include |  UV Content (R1-STU2-ballot) UV Content (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.21.4.19 | Include |  UV Generalized Medicine Class (2023) UV Generalized Medicine Class (2023) | DYNAMIC | | 2.16.840.1.113883.10.21.4.18 | Include |  UV Ingredient (R1-STU2-ballot) UV Ingredient (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.12.151 | Containment |  CDA Organization CDA Organization | DYNAMIC |
|
|
|---|
| Relationship | Version: template 2.16.840.1.113883.10.21.4.11 UV Medication Information (detail) (2017‑05‑10)
Specialization: template 2.16.840.1.113883.10.12.312 CDA ManufacturedProduct (2005‑09‑07) ref ad1bbr-
Adaptation: template 1.3.6.1.4.1.19376.1.9.1.3.1 IHE MedicineEntryContentModule (DYNAMIC) ref ch-pharm- |
|---|
| Item | DT | Card | Conf | Description | Label |
|---|
| | | | | (UVM...ail) |  | @classCode
|
| cs | 1 … 1 | F | MANU |  | hl7:templateId
|
| II | 1 … 1 | M | | (UVM...ail) |  |  | @root
|
| uid | 1 … 1 | F | 2.16.840.1.113883.10.21.4.11 |  | hl7:manufacturedMaterial
|
| | | | | (UVM...ail) |  |  | @classCode
|
| cs | 0 … 1 | F | MMAT |  |  | @determinerCode
|
| cs | 0 … 1 | F | KIND |  |  | hl7:code
|
| CE | 0 … 1 | R | The code describes the code of the medication. The medication may be either
- a brand/product or
- described as a generic/scientific name or
- a descriptor of a magistral preparation/compound medicine
| (UVM...ail) | | ED | 0 … 1 | R | The originalText shoud contain a reference whose URI value points to the name and strength of the medication in the corresponding section.text, or just the name alone if strength is not relevant. | (UVM...ail) | | TEL | 1 … 1 | R | | (UVM...ail) | | CE | 0 … * | | Product code(s) from any organizational or juristdictional system | (UVM...ail) | | | CONF | | shall be drawn from concept domain "Product Code" |
|  |  | hl7:name
|
| EN | 0 … 1 | R | The element SHOULD contain the name of the medication (e.g., "Adol 500mg Caplet"). The medication may be either
- a brand/product or
- described as a generic/scientific name or
- a descriptor of a magistral preparation/compound medicine
| (UVM...ail) |  |  | pharm:desc
|
| ED | 0 … 1 | | | (UVM...ail) |  |  | pharm:formCode
|
| CE | 0 … 1 | | This code represents the pharmaceutical dose form (e.g., tablet, capsule, liquid) and SHOULD be present, if not implied by the product. It MAY be present if implied by the product. The value of this code may affect the units used in the substance administration quantity element. | (UVM...ail) |  |  | hl7:lotNumberText
|
| ST | 0 … 1 | | The lotNumberText element MAY be present and is a string representation of a lot number of this specific instance of the product. The provided lot number SHALL refer to the primary packaged item described in the Packaging element. | (UVM...ail) |  |  | pharm:expirationTime
|
| TS | 0 … 1 | | The pharm:expirationTime element MAY be present and SHALL contain a value attribute containing the date (e.g., specific date, specific date including time) of expiration of this specific instance of the product. The value given in the pharm:expirationTime element SHALL refer to the primary packaged item described in the Medicine Packaging
element. | (UVM...ail) | | | 1 … 1 | R | |  |  | pharm:asContent
|
| | 0 … * | | This structure describes the packaging of the medication. It represents the primary description of the packaging of the medicine (e.g., the medicine is packaged in ampoules of 50ml volume each) and may include additional packaging information of how many of the primary packaged items are within an outer package (e.g., 5 ampoules are packaged in a box).
The primary description of the package should be consistent with the given pharmaceutical dose form (pharm:formCode of the medication). Example: a consistent pharmaceutical dose form to the package form “Ampoules” would be e.g., “Solution for injection”.
In case the package describes a product, the pharm:code element provides the code for the product.
In case the package describes a product, and the package has a brand name, it should be described in the pharm:name element (e.g., Xylocaine 1% with Adrenaline Inj, 5 injections package).
The pharm:formCode element represents the form of the product/contaner (e.g., tablet container, bottle, ...).
The <pharm:capacityQuantity> element describes the capacity of the packaging, while the <pharm:quantity> the actual quantity of inner packaged items in the outer packaging container.
The product might have a single (30 pills bottle) or multiple (5 vials 10 ml; box with 2 blisters of 20 tablets) layers of packaging. In the latter case, the most inner (nested) item represents the most outer package item.
For example the case
\--Box
\-----2 blisters
\--------20 tablets
is described as "20 tablets" contained by "a blister"; "2 blisters" contained by one box.
The most inner package represents the Packaged Medicinal Product.
| (UVM...ail) | | cs | 1 … 1 | F | CONT | | | Example | Packaged Medicinal Product with formCode <asContent classCode="CONT">
<containerPackagedProduct classCode="CONT" determinerCode="KIND">
<!-- Packaged Medicinal Product -->
<code codeSystem="1.999.999" code="PC_ID" displayName="Packaged Product Name"/> <name>100 MIRACLE PILLS(TM)</name> <formCode codeSystem="0.4.0.127.0.16.1.1.2.1" code="30009000" displayName="Box" CodeSystemName="EDQM"/> </containerPackagedProduct></asContent> | | | Example | General example <asContent classCode="CONT">
<quantity value=" " unit=" "/> <containerPackagedProduct classCode="CONT" determinerCode="KIND">
<!-- Medicinal product code (package-level) -->
<code code=" " displayName=" " codeSystem=" " codeSystemName=" "/> <!-- Brand name (package) -->
<name> . . . </name> <formCode code=" " displayName=" " codeSystem=" " codeSystemName=" "/> <capacityQuantity value=" " unit=" "/> <asContent>
<containerPackagedProduct classCode="CONT" determinerCode="KIND">
<capacityQuantity value=" " unit=" "/> </containerPackagedProduct> </asContent> </containerPackagedProduct></asContent> | | | Example | Medicinal product with pharmaceutical dose form ”Tablets”, available as a “Tablet container” with 30 tablets <asContent classCode="CONT">
<!-- 30 tablets in the package -->
<quantity value="30" unit="{tablet}"/> <containerPackagedProduct classCode="CONT" determinerCode="KIND">
<!-- .. -->
<formCode code=" " displayName="Tablet container" codeSystem=" " codeSystemName=" "/> </containerPackagedProduct></asContent> | | | Example | Medicinal product with pharmaceutical dose form 'Solution for injection', available as “Ampoules” with 50ml volume, packaged as 5 ampoules per box <asContent classCode="CONT">
<quantity value="50" unit="ml"/> <!-- 50ml per ampoule -->
<containerPackagedProduct classCode="CONT" determinerCode="KIND">
<!-- .. -->
<formCode code=" " displayName="Ampoules" codeSystem=" " codeSystemName=" "/> <asContent>
<!-- 5 ampoules in a box -->
<quantity value="5"/> <containerPackagedProduct classCode="CONT" determinerCode="KIND">
<!-- .. -->
</containerPackagedProduct> </asContent> </containerPackagedProduct></asContent> | | | Example | Packaged Medicinal Product with multiple layers packaging <asContent classCode="CONT">
<containerPackagedProduct>
<!-- Inner Package -->
<code codeSystem="..." code="..." displayName="..."/> <asContent>
<containerPackagedProduct>
<!-- Intermediate Package -->
<asContent>
<containerPackagedProduct>
<!-- Outer Package / Packaged Medicinal Product -->
</containerPackagedProduct> </asContent> </containerPackagedProduct> </asContent> </containerPackagedProduct></asContent> | | Included | | | from 2.16.840.1.113883.10.21.4.17 UV Content (DYNAMIC) | | PQ | 0 … 1 | R | The quantity which specified how many inner packaged content entities are in an outer packaging container entity.
| (UVM...ail) |  |  |  | pharm:containerPackagedProduct
|
| | 1 … 1 | R | | (UVM...ail) | | cs | 1 … 1 | F | CONT | | cs | 1 … 1 | F | KIND | | CE | 0 … 1 | |
It represents the code of the Package Item or of the Packaged Medicinal Product.
If this is also the most outer <pharm:containerPackagedProduct> than this element can be used to convey the Packaged Medicinal Product ID.
| (UVM...ail) | | EN | 0 … * | |
It represents the Name of the Package Item or of the Packaged Medicinal Product.
If this is also the most outer <pharm:containerPackagedProduct> than this element can be used for the brand name.
| (UVM...ail) | | CE | 0 … 1 | | This element encodes the type of the most inner package item or of the or the Packaged Medicinal Product.
| (UVM...ail) | | PQ | 0 … 1 | | Captures the number of product units the package would contain if fully loaded.
| (UVM...ail) | | | 1 … 1 | R | | | cs | 0 … 1 | | | | | 0 … * | | In case of multiple layers of packaging (5 vials 10 ml; box with 2 blisters of 20 tablets) this element can be used for describing the intermediate Packaged Medicinal Product Item or the Packaged Medicinal Product.
For example in the case
\--Box
\-----2 blisters
\--------20 tablets
it describes the "2 blisters"
In the case of
\--Box
\-----5 vials
it represents the Packaged Medicinal Product. | (UVM...ail) | | cs | 1 … 1 | F | CONT | | PQ | 0 … 1 | R | The quantity which specified how many inner packaged content entities are in an outer packaging container entity.
| (UVM...ail) |  |  |  |  |  | pharm:containerPackagedProduct
|
| | 1 … 1 | R | It represents the intermediate Package Item or the Packaged Medicinal Product
| (UVM...ail) | | cs | 1 … 1 | F | CONT | | cs | 1 … 1 | F | KIND | | CD | 0 … 1 | |
It represents the code of the Package Item or of the Packaged Medicinal Product.
If this is also the most outer <pharm:containerPackagedProduct> than this element can be used to convey the Packaged Medicinal Product ID.
| (UVM...ail) | | ST | 0 … 1 | R | It represents the Name of the Package Item or of the Packaged Medicinal Product.
If this is also the most outer <pharm:containerPackagedProduct> than this element can be used for the brand name. | (UVM...ail) | | CE | 1 … 1 | R | This element encodes the type of the intermediate package item or of the or the Packaged Medicinal Product.
| (UVM...ail) | | PQ | 0 … 1 | | Captures the number of product units the package would contain if fully loaded.
| (UVM...ail) | | | 1 … 1 | R | | | cs | 0 … 1 | | | | | 0 … * | R |
In case of multiple layers of packaging (box with 2 blisters of 20 tablets) this element is used for describing the most outer Packaged Medicinal Product Item or the Packaged Medicinal Product.
For example in the case
\--Box
\-----2 blisters
\--------20 tablets
it describes the Packaged Medicinal Product.
| (UVM...ail) | | cs | 1 … 1 | F | CONT | | PQ | 0 … 1 | R | | (UVM...ail) |  |  |  |  |  |  |  | pharm:containerPackagedProduct
|
| | 1 … 1 | R | When present, it represents the Packaged Medicinal Product | (UVM...ail) | | cs | 1 … 1 | F | CONT | | cs | 1 … 1 | F | KIND | | CD | 0 … 1 | | When present, it can be used to convey the Packaged Medicinal Product ID.
| (UVM...ail) | | ST | 0 … 1 | R | | (UVM...ail) | | CE | 1 … 1 | R | | (UVM...ail) | | PQ | 0 … 1 | | Captures the number of product units the package would contain if fully loaded.
| (UVM...ail) | | | 1 … 1 | R | | | cs | 0 … 1 | | |  |  | pharm:asSpecializedKind
|
| | 0 … * | R |
The Medicinal Product can be classified according to various classification systems, which may be jurisdictional or international as for example the WHO ATC drug code, or the IDMP Pharmaceutical Product Identifier(s) (PhPID Set) when it will be available for use.
The generalizedMaterialKind/code element is used to covey these codes.
| (UVM...ail) | | cs | 1 … 1 | F | GRIC | | | Example | <asSpecializedKind classCode="GRIC">
<generalizedMedicineClass classCode="MMAT">
<code code=" " displayName=" " codeSystem=" " codeSystemName=" "/> </generalizedMedicineClass></asSpecializedKind> | | Included | | | from 2.16.840.1.113883.10.21.4.19 UV Generalized Medicine Class (DYNAMIC) |  |  |  | pharm:generalizedMedicineKind
|
| | 0 … * | | | (UVM...ail) | | cs | 1 … 1 | F | MMAT | | cs | 1 … 1 | F | KIND | | | 1 … 1 | R | | (UVM...ail) | | | 0 … * | | | (UVM...ail) |  |  | pharm:part
|
| | 0 … * | | | (UVM...ail) | | cs | 1 … 1 | F | PART | | II | 0 … 1 | | | (UVM...ail) | | PQ | 0 … 1 | | | (UVM...ail) | | | 1 … 1 | | | (UVM...ail) | | CE | 0 … 1 | | | (UVM...ail) | | EN | 0 … 1 | | | (UVM...ail) | | ED | 0 … 1 | | | (UVM...ail) | | CE | 0 … 1 | | | (UVM...ail) | | | 0 … * | | | (UVM...ail) | | cs | 1 … 1 | F | CONT | | Included | | | from 2.16.840.1.113883.10.21.4.17 UV Content (DYNAMIC) | | PQ | 0 … 1 | R | The quantity which specified how many inner packaged content entities are in an outer packaging container entity.
| (UVM...ail) |  |  |  |  |  | pharm:containerPackagedProduct
|
| | 1 … 1 | R | | (UVM...ail) | | cs | 1 … 1 | F | CONT | | cs | 1 … 1 | F | KIND | | CE | 0 … 1 | |
It represents the code of the Package Item or of the Packaged Medicinal Product.
If this is also the most outer <pharm:containerPackagedProduct> than this element can be used to convey the Packaged Medicinal Product ID.
| (UVM...ail) | | EN | 0 … * | |
It represents the Name of the Package Item or of the Packaged Medicinal Product.
If this is also the most outer <pharm:containerPackagedProduct> than this element can be used for the brand name.
| (UVM...ail) | | CE | 0 … 1 | | This element encodes the type of the most inner package item or of the or the Packaged Medicinal Product.
| (UVM...ail) | | PQ | 0 … 1 | | Captures the number of product units the package would contain if fully loaded.
| (UVM...ail) | | | 1 … 1 | R | | | cs | 0 … 1 | | | | | 0 … * | | In case of multiple layers of packaging (5 vials 10 ml; box with 2 blisters of 20 tablets) this element can be used for describing the intermediate Packaged Medicinal Product Item or the Packaged Medicinal Product.
For example in the case
\--Box
\-----2 blisters
\--------20 tablets
it describes the "2 blisters"
In the case of
\--Box
\-----5 vials
it represents the Packaged Medicinal Product. | (UVM...ail) | | cs | 1 … 1 | F | CONT | | PQ | 0 … 1 | R | The quantity which specified how many inner packaged content entities are in an outer packaging container entity.
| (UVM...ail) |  |  |  |  |  |  |  | pharm:containerPackagedProduct
|
| | 1 … 1 | R | It represents the intermediate Package Item or the Packaged Medicinal Product
| (UVM...ail) | | cs | 1 … 1 | F | CONT | | cs | 1 … 1 | F | KIND | | CD | 0 … 1 | |
It represents the code of the Package Item or of the Packaged Medicinal Product.
If this is also the most outer <pharm:containerPackagedProduct> than this element can be used to convey the Packaged Medicinal Product ID.
| (UVM...ail) | | ST | 0 … 1 | R | It represents the Name of the Package Item or of the Packaged Medicinal Product.
If this is also the most outer <pharm:containerPackagedProduct> than this element can be used for the brand name. | (UVM...ail) | | CE | 1 … 1 | R | This element encodes the type of the intermediate package item or of the or the Packaged Medicinal Product.
| (UVM...ail) | | PQ | 0 … 1 | | Captures the number of product units the package would contain if fully loaded.
| (UVM...ail) | | | 1 … 1 | R | | | cs | 0 … 1 | | | | | 0 … * | R |
In case of multiple layers of packaging (box with 2 blisters of 20 tablets) this element is used for describing the most outer Packaged Medicinal Product Item or the Packaged Medicinal Product.
For example in the case
\--Box
\-----2 blisters
\--------20 tablets
it describes the Packaged Medicinal Product.
| (UVM...ail) | | cs | 1 … 1 | F | CONT | | PQ | 0 … 1 | R | | (UVM...ail) |  |  |  |  |  |  |  |  |  | pharm:containerPackagedProduct
|
| | 1 … 1 | R | When present, it represents the Packaged Medicinal Product | (UVM...ail) | | cs | 1 … 1 | F | CONT | | cs | 1 … 1 | F | KIND | | CD | 0 … 1 | | When present, it can be used to convey the Packaged Medicinal Product ID.
| (UVM...ail) | | ST | 0 … 1 | R | | (UVM...ail) | | CE | 1 … 1 | R | | (UVM...ail) | | PQ | 0 … 1 | | Captures the number of product units the package would contain if fully loaded.
| (UVM...ail) | | | 1 … 1 | R | | | cs | 0 … 1 | | | | | 0 … * | | | (UVM...ail) | | cs | 1 … 1 | F | GRIC | | Included | | | from 2.16.840.1.113883.10.21.4.19 UV Generalized Medicine Class (DYNAMIC) |  |  |  |  |  | pharm:generalizedMedicineKind
|
| | 0 … * | | | (UVM...ail) | | cs | 1 … 1 | F | MMAT | | cs | 1 … 1 | F | KIND | | | 1 … 1 | R | | (UVM...ail) | | | 0 … * | | | (UVM...ail) | | | 0 … * | | | (UVM...ail) | | cs | 1 … 1 | F | PART | | II | 0 … 1 | | | (UVM...ail) | | PQ | 0 … 1 | | | (UVM...ail) | | | 1 … 1 | | | (UVM...ail) | | CE | 0 … 1 | | | (UVM...ail) | | EN | 0 … 1 | | | (UVM...ail) | | ED | 0 … 1 | | | (UVM...ail) | | CE | 0 … 1 | | | (UVM...ail) | | | 0 … * | | | (UVM...ail) | | cs | 1 … 1 | F | CONT | | Included | | | from 2.16.840.1.113883.10.21.4.17 UV Content (DYNAMIC) | | PQ | 0 … 1 | R | The quantity which specified how many inner packaged content entities are in an outer packaging container entity.
| (UVM...ail) |  |  |  |  |  |  |  | pharm:containerPackagedProduct
|
| | 1 … 1 | R | | (UVM...ail) | | cs | 1 … 1 | F | CONT | | cs | 1 … 1 | F | KIND | | CE | 0 … 1 | |
It represents the code of the Package Item or of the Packaged Medicinal Product.
If this is also the most outer <pharm:containerPackagedProduct> than this element can be used to convey the Packaged Medicinal Product ID.
| (UVM...ail) | | EN | 0 … * | |
It represents the Name of the Package Item or of the Packaged Medicinal Product.
If this is also the most outer <pharm:containerPackagedProduct> than this element can be used for the brand name.
| (UVM...ail) | | CE | 0 … 1 | | This element encodes the type of the most inner package item or of the or the Packaged Medicinal Product.
| (UVM...ail) | | PQ | 0 … 1 | | Captures the number of product units the package would contain if fully loaded.
| (UVM...ail) | | | 1 … 1 | R | | | cs | 0 … 1 | | | | | 0 … * | | In case of multiple layers of packaging (5 vials 10 ml; box with 2 blisters of 20 tablets) this element can be used for describing the intermediate Packaged Medicinal Product Item or the Packaged Medicinal Product.
For example in the case
\--Box
\-----2 blisters
\--------20 tablets
it describes the "2 blisters"
In the case of
\--Box
\-----5 vials
it represents the Packaged Medicinal Product. | (UVM...ail) | | cs | 1 … 1 | F | CONT | | PQ | 0 … 1 | R | The quantity which specified how many inner packaged content entities are in an outer packaging container entity.
| (UVM...ail) |  |  |  |  |  |  |  |  |  | pharm:containerPackagedProduct
|
| | 1 … 1 | R | It represents the intermediate Package Item or the Packaged Medicinal Product
| (UVM...ail) | | cs | 1 … 1 | F | CONT | | cs | 1 … 1 | F | KIND | | CD | 0 … 1 | |
It represents the code of the Package Item or of the Packaged Medicinal Product.
If this is also the most outer <pharm:containerPackagedProduct> than this element can be used to convey the Packaged Medicinal Product ID.
| (UVM...ail) | | ST | 0 … 1 | R | It represents the Name of the Package Item or of the Packaged Medicinal Product.
If this is also the most outer <pharm:containerPackagedProduct> than this element can be used for the brand name. | (UVM...ail) | | CE | 1 … 1 | R | This element encodes the type of the intermediate package item or of the or the Packaged Medicinal Product.
| (UVM...ail) | | PQ | 0 … 1 | | Captures the number of product units the package would contain if fully loaded.
| (UVM...ail) | | | 1 … 1 | R | | | cs | 0 … 1 | | | | | 0 … * | R |
In case of multiple layers of packaging (box with 2 blisters of 20 tablets) this element is used for describing the most outer Packaged Medicinal Product Item or the Packaged Medicinal Product.
For example in the case
\--Box
\-----2 blisters
\--------20 tablets
it describes the Packaged Medicinal Product.
| (UVM...ail) | | cs | 1 … 1 | F | CONT | | PQ | 0 … 1 | R | | (UVM...ail) |  |  |  |  |  |  |  |  |  |  |  | pharm:containerPackagedProduct
|
| | 1 … 1 | R | When present, it represents the Packaged Medicinal Product | (UVM...ail) | | cs | 1 … 1 | F | CONT | | cs | 1 … 1 | F | KIND | | CD | 0 … 1 | | When present, it can be used to convey the Packaged Medicinal Product ID.
| (UVM...ail) | | ST | 0 … 1 | R | | (UVM...ail) | | CE | 1 … 1 | R | | (UVM...ail) | | PQ | 0 … 1 | | Captures the number of product units the package would contain if fully loaded.
| (UVM...ail) | | | 1 … 1 | R | | | cs | 0 … 1 | | | | | 0 … * | | | (UVM...ail) | | cs | 1 … 1 | F | GRIC | | Included | | | from 2.16.840.1.113883.10.21.4.19 UV Generalized Medicine Class (DYNAMIC) |  |  |  |  |  |  |  | pharm:generalizedMedicineKind
|
| | 0 … * | | | (UVM...ail) | | cs | 1 … 1 | F | MMAT | | cs | 1 … 1 | F | KIND | | | 1 … 1 | R | | (UVM...ail) | | | 0 … * | | | (UVM...ail) | | | 0 … * | | | (UVM...ail) | | cs | 1 … 1 | F | PART | | II | 0 … 1 | | | (UVM...ail) | | PQ | 0 … 1 | | | (UVM...ail) | | | 1 … 1 | | | (UVM...ail) | | CE | 0 … 1 | | | (UVM...ail) | | EN | 0 … 1 | | | (UVM...ail) | | ED | 0 … 1 | | | (UVM...ail) | | CE | 0 … 1 | | | (UVM...ail) | | | 0 … * | | | (UVM...ail) | | cs | 1 … 1 | F | CONT | | Included | | | from 2.16.840.1.113883.10.21.4.17 UV Content (DYNAMIC) | | PQ | 0 … 1 | R | The quantity which specified how many inner packaged content entities are in an outer packaging container entity.
| (UVM...ail) |  |  |  |  |  |  |  |  |  | pharm:containerPackagedProduct
|
| | 1 … 1 | R | | (UVM...ail) | | cs | 1 … 1 | F | CONT | | cs | 1 … 1 | F | KIND | | CE | 0 … 1 | |
It represents the code of the Package Item or of the Packaged Medicinal Product.
If this is also the most outer <pharm:containerPackagedProduct> than this element can be used to convey the Packaged Medicinal Product ID.
| (UVM...ail) | | EN | 0 … * | |
It represents the Name of the Package Item or of the Packaged Medicinal Product.
If this is also the most outer <pharm:containerPackagedProduct> than this element can be used for the brand name.
| (UVM...ail) | | CE | 0 … 1 | | This element encodes the type of the most inner package item or of the or the Packaged Medicinal Product.
| (UVM...ail) | | PQ | 0 … 1 | | Captures the number of product units the package would contain if fully loaded.
| (UVM...ail) | | | 1 … 1 | R | | | cs | 0 … 1 | | | | | 0 … * | | In case of multiple layers of packaging (5 vials 10 ml; box with 2 blisters of 20 tablets) this element can be used for describing the intermediate Packaged Medicinal Product Item or the Packaged Medicinal Product.
For example in the case
\--Box
\-----2 blisters
\--------20 tablets
it describes the "2 blisters"
In the case of
\--Box
\-----5 vials
it represents the Packaged Medicinal Product. | (UVM...ail) | | cs | 1 … 1 | F | CONT | | PQ | 0 … 1 | R | The quantity which specified how many inner packaged content entities are in an outer packaging container entity.
| (UVM...ail) |  |  |  |  |  |  |  |  |  |  |  | pharm:containerPackagedProduct
|
| | 1 … 1 | R | It represents the intermediate Package Item or the Packaged Medicinal Product
| (UVM...ail) | | cs | 1 … 1 | F | CONT | | cs | 1 … 1 | F | KIND | | CD | 0 … 1 | |
It represents the code of the Package Item or of the Packaged Medicinal Product.
If this is also the most outer <pharm:containerPackagedProduct> than this element can be used to convey the Packaged Medicinal Product ID.
| (UVM...ail) | | ST | 0 … 1 | R | It represents the Name of the Package Item or of the Packaged Medicinal Product.
If this is also the most outer <pharm:containerPackagedProduct> than this element can be used for the brand name. | (UVM...ail) | | CE | 1 … 1 | R | This element encodes the type of the intermediate package item or of the or the Packaged Medicinal Product.
| (UVM...ail) | | PQ | 0 … 1 | | Captures the number of product units the package would contain if fully loaded.
| (UVM...ail) | | | 1 … 1 | R | | | cs | 0 … 1 | | | | | 0 … * | R |
In case of multiple layers of packaging (box with 2 blisters of 20 tablets) this element is used for describing the most outer Packaged Medicinal Product Item or the Packaged Medicinal Product.
For example in the case
\--Box
\-----2 blisters
\--------20 tablets
it describes the Packaged Medicinal Product.
| (UVM...ail) | | cs | 1 … 1 | F | CONT | | PQ | 0 … 1 | R | | (UVM...ail) |  |  |  |  |  |  |  |  |  |  |  |  |  | pharm:containerPackagedProduct
|
| | 1 … 1 | R | When present, it represents the Packaged Medicinal Product | (UVM...ail) | | cs | 1 … 1 | F | CONT | | cs | 1 … 1 | F | KIND | | CD | 0 … 1 | | When present, it can be used to convey the Packaged Medicinal Product ID.
| (UVM...ail) | | ST | 0 … 1 | R | | (UVM...ail) | | CE | 1 … 1 | R | | (UVM...ail) | | PQ | 0 … 1 | | Captures the number of product units the package would contain if fully loaded.
| (UVM...ail) | | | 1 … 1 | R | | | cs | 0 … 1 | | | | | 0 … * | | | (UVM...ail) | | cs | 1 … 1 | F | GRIC | | Included | | | from 2.16.840.1.113883.10.21.4.19 UV Generalized Medicine Class (DYNAMIC) |  |  |  |  |  |  |  |  |  | pharm:generalizedMedicineKind
|
| | 0 … * | | | (UVM...ail) | | cs | 1 … 1 | F | MMAT | | cs | 1 … 1 | F | KIND | | | 1 … 1 | R | | (UVM...ail) | | | 0 … * | | | (UVM...ail) | | | 0 … * | | | (UVM...ail) | | cs | 1 … 1 | F | PART | | | 0 … * | | | (UVM...ail) | | cs | 1 … 1 | R | | | | CONF | | The value of @classCode shall be drawn from value set 2.16.840.1.113883.1.11.10430 RoleClassIngredientEntity (DYNAMIC) |
| | Included | | | from 2.16.840.1.113883.10.21.4.18 UV Ingredient (DYNAMIC) | | RTO_PQ_PQ | 0 … 1 | | | (UVM...ail) | | | Example | 10 mg of the ingredient per ml <pharm:quantity>
<cda:numerator xsi:type="PQ" value="10" unit="mg"/> <cda:denominator xsi:type="PQ" value="1" unit="ml"/></pharm:quantity> | | | Example | 2% of the ingredient <pharm:quantity>
<cda:numerator xsi:type="PQ" value="2" unit="%"/> <cda:denominator xsi:type="PQ" value="1"/></pharm:quantity> | | | Example | 5mg of the ingredient <pharm:quantity>
<cda:numerator xsi:type="PQ" value="5" unit="mg"/> <cda:denominator xsi:type="PQ" value="1"/></pharm:quantity> | | PQ | 0 … 1 | | | (UVM...ail) | | PQ | 0 … 1 | | | (UVM...ail) |  |  |  |  |  |  |  |  |  | pharm:ingredientSubstance
|
| | 0 … 1 | | The substance used for this product plying the role indicated in the ingredient classCode.
The <code> element contains the coded representation of the ingredient and the <name> element may be used for the plain text representation.
| (UVM...ail) | | cs | 1 … 1 | F | MMAT | | cs | 1 … 1 | F | KIND | | CD | 0 … 1 | C | | (UVM...ail) | | EN | 0 … 1 | C | | (UVM...ail) | | | Schematron assert | role | error | | | | test | pharm:code or pharm:name | | | | Message | Either the name or the code of the substance (or both) shall be provided | | | | 0 … * | | | (UVM...ail) | | cs | 1 … 1 | R | | | | CONF | | The value of @classCode shall be drawn from value set 2.16.840.1.113883.1.11.10430 RoleClassIngredientEntity (DYNAMIC) |
| | Included | | | from 2.16.840.1.113883.10.21.4.18 UV Ingredient (DYNAMIC) | | RTO_PQ_PQ | 0 … 1 | | | (UVM...ail) | | | Example | 10 mg of the ingredient per ml <pharm:quantity>
<cda:numerator xsi:type="PQ" value="10" unit="mg"/> <cda:denominator xsi:type="PQ" value="1" unit="ml"/></pharm:quantity> | | | Example | 2% of the ingredient <pharm:quantity>
<cda:numerator xsi:type="PQ" value="2" unit="%"/> <cda:denominator xsi:type="PQ" value="1"/></pharm:quantity> | | | Example | 5mg of the ingredient <pharm:quantity>
<cda:numerator xsi:type="PQ" value="5" unit="mg"/> <cda:denominator xsi:type="PQ" value="1"/></pharm:quantity> | | PQ | 0 … 1 | | | (UVM...ail) | | PQ | 0 … 1 | | | (UVM...ail) |  |  |  |  |  |  |  | pharm:ingredientSubstance
|
| | 0 … 1 | | The substance used for this product plying the role indicated in the ingredient classCode.
The <code> element contains the coded representation of the ingredient and the <name> element may be used for the plain text representation.
| (UVM...ail) | | cs | 1 … 1 | F | MMAT | | cs | 1 … 1 | F | KIND | | CD | 0 … 1 | C | | (UVM...ail) | | EN | 0 … 1 | C | | (UVM...ail) | | | Schematron assert | role | error | | | | test | pharm:code or pharm:name | | | | Message | Either the name or the code of the substance (or both) shall be provided | | | | 0 … * | | | (UVM...ail) | | cs | 1 … 1 | R | | | | CONF | | The value of @classCode shall be drawn from value set 2.16.840.1.113883.1.11.10430 RoleClassIngredientEntity (DYNAMIC) |
| | Included | | | from 2.16.840.1.113883.10.21.4.18 UV Ingredient (DYNAMIC) | | RTO_PQ_PQ | 0 … 1 | | | (UVM...ail) | | | Example | 10 mg of the ingredient per ml <pharm:quantity>
<cda:numerator xsi:type="PQ" value="10" unit="mg"/> <cda:denominator xsi:type="PQ" value="1" unit="ml"/></pharm:quantity> | | | Example | 2% of the ingredient <pharm:quantity>
<cda:numerator xsi:type="PQ" value="2" unit="%"/> <cda:denominator xsi:type="PQ" value="1"/></pharm:quantity> | | | Example | 5mg of the ingredient <pharm:quantity>
<cda:numerator xsi:type="PQ" value="5" unit="mg"/> <cda:denominator xsi:type="PQ" value="1"/></pharm:quantity> | | PQ | 0 … 1 | | | (UVM...ail) | | PQ | 0 … 1 | | | (UVM...ail) |  |  |  |  |  | pharm:ingredientSubstance
|
| | 0 … 1 | | The substance used for this product plying the role indicated in the ingredient classCode.
The <code> element contains the coded representation of the ingredient and the <name> element may be used for the plain text representation.
| (UVM...ail) | | cs | 1 … 1 | F | MMAT | | cs | 1 … 1 | F | KIND | | CD | 0 … 1 | C | | (UVM...ail) | | EN | 0 … 1 | C | | (UVM...ail) | | | Schematron assert | role | error | | | | test | pharm:code or pharm:name | | | | Message | Either the name or the code of the substance (or both) shall be provided | |  |  | pharm:ingredient
|
| | 0 … * | |
This module provides the list of the ingredients (substances with a role) used for this product; one or more ingredients may be present.
The classCode of "ACTI" indicates that this is an active ingredient.
| (UVM...ail) | | cs | 1 … 1 | R | | | | CONF | | The value of @classCode shall be drawn from value set 2.16.840.1.113883.1.11.10430 RoleClassIngredientEntity (DYNAMIC) |
| | | Example | <pharm:ingredient classCode="ACTI">
<pharm:quantity>
<cda:numerator type="PQ" value=" " unit=" "/> <cda:denominator type="PQ" value=" " unit=" "/> </pharm:quantity> <ingredientSubstance classCode="MMAT" determinerCode="KIND">
<code code=" " displayName=" " codeSystem="2.16.840.1.113883.6.73" codeSystemName="ATC WHO"/> </ingredientSubstance></pharm:ingredient> | | Included | | | from 2.16.840.1.113883.10.21.4.18 UV Ingredient (DYNAMIC) | | RTO_PQ_PQ | 0 … 1 | | | (UVM...ail) | | | Example | 10 mg of the ingredient per ml <pharm:quantity>
<cda:numerator xsi:type="PQ" value="10" unit="mg"/> <cda:denominator xsi:type="PQ" value="1" unit="ml"/></pharm:quantity> | | | Example | 2% of the ingredient <pharm:quantity>
<cda:numerator xsi:type="PQ" value="2" unit="%"/> <cda:denominator xsi:type="PQ" value="1"/></pharm:quantity> | | | Example | 5mg of the ingredient <pharm:quantity>
<cda:numerator xsi:type="PQ" value="5" unit="mg"/> <cda:denominator xsi:type="PQ" value="1"/></pharm:quantity> | | PQ | 0 … 1 | | | (UVM...ail) | | PQ | 0 … 1 | | | (UVM...ail) |  |  |  | pharm:ingredientSubstance
|
| | 0 … 1 | | The substance used for this product plying the role indicated in the ingredient classCode.
The <code> element contains the coded representation of the ingredient and the <name> element may be used for the plain text representation.
| (UVM...ail) | | cs | 1 … 1 | F | MMAT | | cs | 1 … 1 | F | KIND | | CD | 0 … 1 | C | | (UVM...ail) | | EN | 0 … 1 | C | | (UVM...ail) | | | Schematron assert | role | error | | | | test | pharm:code or pharm:name | | | | Message | Either the name or the code of the substance (or both) shall be provided | |  | hl7:manufacturerOrganization
|
| | 0 … 1 | R | Contains 2.16.840.1.113883.10.12.151 CDA Organization (DYNAMIC) | (UVM...ail) |
|
UV Medication Information (simple)
| Id | 2.16.840.1.113883.10.21.4.10 | Effective Date | 2021‑09‑29 19:15:16Other versions this id:  UVMedicationInformationsimple as of 2021‑09‑29 19:15:02 UVMedicationInformationsimple as of 2021‑09‑29 19:15:02 UVMedicationInformationsimple as of 2017‑05‑10 UVMedicationInformationsimple as of 2017‑05‑10
|
|---|
| Status |  Under pre-publication review Under pre-publication review | Version Label | R1-STU2-ballot |
|---|
| Name | UVMedicationInformationsimple | Display Name | UV Medication Information (simple) |
|---|
| Description | Universal Medication Information (simple) |
|---|
| Context | Parent nodes of template element with id 2.16.840.1.113883.10.21.4.10 |
|---|
| Classification | CDA Entry Level Template |
|---|
| Open/Closed | Open (other than defined elements are allowed) |
|---|
| Uses | | Uses 1 template | | Uses | as | Name | Version |
|---|
| 2.16.840.1.113883.10.12.151 | Containment |  CDA Organization CDA Organization | DYNAMIC |
|
|
|---|
| Relationship | Version: template 2.16.840.1.113883.10.21.4.10 UV Medication Information (simple) (2017‑05‑10)
Specialization: template 2.16.840.1.113883.10.12.312 CDA ManufacturedProduct (2005‑09‑07) ref ad1bbr-
Adaptation: template 2.16.840.1.113883.10.20.22.4.54 Immunization Medication Information (V2) (2014‑06‑09) ref ccda- |
|---|
| Example | | US RxNorm Code | <hl7:manufacturedProduct classCode="MANU">
<hl7:templateId root="2.16.840.1.113883.10.21.4.10"/> <hl7:manufacturedMaterial classCode="MMAT" determinerCode="KIND">
<hl7:code code="243670" codeSystem="2.16.840.1.113883.6.88" displayName="Aspirin 81 MG Oral Tablet"/> </hl7:manufacturedMaterial></hl7:manufacturedProduct> |
|
|---|
| Example | | Dutch G-Standaard Artikel Code | <hl7:manufacturedProduct classCode="MANU">
<hl7:templateId root="2.16.840.1.113883.10.21.4.10"/> <hl7:manufacturedMaterial classCode="MMAT" determinerCode="KIND">
<hl7:code code="14145839" codeSystem="2.16.840.1.113883.2.4.4.8" codeSystemName="G-Standaard Artikel" displayName="FUROSEMIDE CF 40MG TABLET"/> </hl7:manufacturedMaterial></hl7:manufacturedProduct> |
|
|---|
| Example | | German Pharmaceutical Product Code | <hl7:manufacturedProduct classCode="MANU">
<hl7:templateId root="2.16.840.1.113883.10.21.4.10"/> <hl7:manufacturedMaterial classCode="MMAT" determinerCode="KIND">
<hl7:code code="4213974" codeSystem="1.2.276.0.76.4.6" displayName="RAMIPRIL STADA 5 mg"/> <hl7:lotNumberText>675-86574</hl7:lotNumberText> </hl7:manufacturedMaterial> <hl7:manufacturerOrganization>
<hl7:name>STADA GmbH</hl7:name> </hl7:manufacturerOrganization></hl7:manufacturedProduct> |
|
|---|
|
UV Medication Order Reference
UV Subordinate Substance Administration
| Id | 2.16.840.1.113883.10.21.4.6 | Effective Date | 2023‑01‑30 09:36:00Other versions this id:  UVSubordinateadministration as of 2017‑04‑30 UVSubordinateadministration as of 2017‑04‑30
|
|---|
| Status |  Draft Draft | Version Label | 2023 |
|---|
| Name | UVSubordinateadministration | Display Name | UV Subordinate Substance Administration |
|---|
| Description | Universal Subordinate Substance Administration to convey information about dosages |
|---|
| Context | Parent nodes of template element with id 2.16.840.1.113883.10.21.4.6 |
|---|
| Classification | CDA Entry Level Template |
|---|
| Open/Closed | Open (other than defined elements are allowed) |
|---|
| Relationship | Version: template 2.16.840.1.113883.10.21.4.6 UV Subordinate Substance Administration (2017‑04‑30)
Specialization: template 2.16.840.1.113883.10.12.308 CDA SubstanceAdministration (2005‑09‑07) ref ad1bbr- |
|---|
| Example | | Example | <substanceAdministration classCode="SBADM" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.21.4.6"/> <statusCode code="active"/> <effectiveTime xsi:type="PIVL_TS" institutionSpecified="true">
<period value="12" unit="h"/> </effectiveTime> <doseQuantity xsi:type="IVL_PQ" value="2" unit="{puff}"/> <consumable>
<manufacturedProduct>
<manufacturedMaterial nullFlavor="NA"/> </manufacturedProduct> </consumable></substanceAdministration> |
|
|---|
| Item | DT | Card | Conf | Description | Label |
|---|
hl7:substanceAdministration
|
| | 1 … 1 | R | | (UVS...ion) |  | @classCode
|
| cs | 1 … 1 | F | SBADM |  | @moodCode
|
| cs | 1 … 1 | R | If the subordinate substance administration refers to Medication Order then a substance administration request (moodCode is 'RQO') is used.
If it refers to a Medication Statement, the moodCode shall be set to event/intent (moodCode is 'EVN' or 'INT'). | | | CONF | | The value of @moodCode shall be drawn from value set 2.16.840.1.113883.11.21.4 Mood Code Evn Int Rqo (DYNAMIC) |
| | | Constraint | The moodCode of this subordinate substance administration SHALL be the same of the parent substance administration |  | hl7:templateId
|
| II | 1 … 1 | M | | (UVS...ion) |  |  | @root
|
| uid | 1 … 1 | F | 2.16.840.1.113883.10.21.4.6 |  | hl7:statusCode
|
| CS | 1 … 1 | M | | (UVS...ion) | | | Constraint | The statusCode of this subordinate substance administration SHALL be the same of that of the parent substance administration. | | | CONF | | The value of @code shall be drawn from value set 2.16.840.1.113883.11.21.2 ActStatusActiveCompletedAbortedSuspended (DYNAMIC) |
| | Choice | 1 … 1 | | Elements to choose from:- hl7:effectiveTime[@value or @nullFlavor]
- hl7:effectiveTime[@xsi:type='PIVL_TS']
- hl7:effectiveTime[@xsi:type='EIVL_TS']
- hl7:effectiveTime[@xsi:type='SXPR_TS']
|  |  | hl7:effectiveTime
|
| TS | 0 … 1 | C | This required element describes the frequency of intakes. If not known it shall be valued with the nullflavor "UNK" | (UVS...ion) | | where [@value or @nullFlavor] | | | | Example | Once (known date) <effectiveTime value="20170404"/> | | | Example | Unknown <effectiveTime nullFlavor="UNK"/> |  |  | hl7:effectiveTime
|
| PIVL_TS | 0 … 1 | C | Periodic Time Interval | (UVS...ion) | | where [@xsi:type='PIVL_TS'] | | | | Example | Every 4 hours <effectiveTime xsi:type="PIVL_TS" institutionSpecified="false">
<period value="4" unit="h"/></effectiveTime> | | | Example | Twice a day <effectiveTime xsi:type="PIVL_TS" institutionSpecified="true">
<period value="12" unit="h"/></effectiveTime> |  |  | hl7:effectiveTime
|
| EIVL_TS | 0 … 1 | C | Event Related Time Interval | (UVS...ion) | | where [@xsi:type='EIVL_TS'] | | | | Example | After meal <effectiveTime xsi:type="EIVL_TS">
<event code="PC" codeSystem="2.16.840.1.113883.5.139"/></effectiveTime> | | | Example | One hour before breakfast <effectiveTime xsi:type="EIVL_TS">
<event code="ACM" codeSystem="2.16.840.1.113883.5.139"/> <offset>
<low value="1" unit="h"/> </offset></effectiveTime> | | EIVL.event | 0 … 1 | C | | (UVS...ion) | | cs | 0 … 1 | | | | | CONF | | The value of @code shall be drawn from value set 2.16.840.1.113883.1.11.10706 TimingEvent (DYNAMIC) |
|  |  | hl7:effectiveTime
|
| SXPR_TS | 0 … 1 | R | Combined Time Interval | (UVS...ion) | | where [@xsi:type='SXPR_TS'] | |  | hl7:doseQuantity
|
| IVL_PQ | 0 … 1 | R | The doseQuantity describes the amount of the medication given (the dosage).
If a dose range is given (e.g., 1-2 tablets, or 325-750mg), then the <low> and <high> bounds are specified in their respective elements; otherwise only one physical quantity is specified (e.g. 2 drops)
The dose can be in some known and measurable unit, such as grams, milligrams,or described in "administration" units (unit of presentation, such as capsules).
If the dose is in countable items (tablets, caplets, "eaches"), then the unit could be omitted or valorized using the UCUM annotations for describing the type of countable items (e.g. .{tablet}, {puff},..).
The unit attribute – when expresses unit of measures- shall be derived from the UCUM code system.
The used elements should contain a <translation> element that provides a reference to the originalText found in the narrative body of the document. | (UVS...ion) |  |  | @unit
|
| cs | 0 … 1 | | | | | Example | Not pre-coordinated consumable <doseQuantity value="25" unit="mg"/> | | | Example | Pre-coordinated consumable - Dose Range <doseQuantity>
<low value="1" unit="{tablet}"/> <high value="2" unit="{tablet}"/></doseQuantity> | | | Example | Pre-coordinated consumable <doseQuantity value="2" unit="{puff}"/> | | | Example | Pre-coordinated consumable with text reference <doseQuantity value="2" unit="{puff}">
<translation nullFlavor="NI">
<originalText>
<reference value="#text-ref-1"/> </originalText> </translation></doseQuantity> | | | Example | Textual dosage <doseQuantity nullFlavor="NI">
<translation nullFlavor="NI">
<originalText>
<reference value="#text-ref-1"/> </originalText> </translation></doseQuantity> |  | hl7:rateQuantity
|
| IVL_PQ | 0 … 1 | | | (UVS...ion) |  | hl7:maxDoseQuantity
|
| RTO_PQ_PQ | 0 … 1 | | | (UVS...ion) |  | hl7:administrationUnitCode
|
| CE | 0 … 1 | | | (UVS...ion) | | | CONF | | The value of @code shall be drawn from value set 2.16.840.1.113883.1.11.14570 AdministrableDrugForm (DYNAMIC) |
|  | hl7:consumable
|
| | 1 … 1 | R | | (UVS...ion) |  |  | hl7:manufacturedProduct
|
| | 1 … 1 | R | | (UVS...ion) |  |  |  | hl7:manufacturedMaterial
|
| | 1 … 1 | R | | (UVS...ion) | | cs | 1 … 1 | F | NA |
|
UV Substitution Event Adminstration
| Id | 2.16.840.1.113883.10.21.4.14 | Effective Date | 2023‑01‑30 08:42:54Other versions this id:  UVSubstitutionEventAdministration as of 2019‑02‑17 UVSubstitutionEventAdministration as of 2019‑02‑17
|
|---|
| Status |  Draft Draft | Version Label | 2023 |
|---|
| Name | UVSubstitutionEventAdministration | Display Name | UV Substitution Event Adminstration |
|---|
| Description | Information about a substitution made for this adminstration. |
|---|
| Context | Parent nodes of template element with id 2.16.840.1.113883.10.21.4.14 |
|---|
| Classification | CDA Entry Level Template |
|---|
| Open/Closed | Open (other than defined elements are allowed) |
|---|
| Relationship | Version: template 2.16.840.1.113883.10.21.4.14 UV Substitution Event Adminstration (2019‑02‑17)
Specialization: template 2.16.840.1.113883.10.12.301 CDA Act (2005‑09‑07) ref ad1bbr- |
|---|
| Example | | Example | <act classCode="ACT" moodCode="EVN">
<templateId root="2.16.840.1.113883.10.21.4.14"/> <code code="TE" codeSystem="2.16.840.1.113883.5.1070" displayName="therapeutic alternative"/> <entryRelationship typeCode="RSON">
<act classCode="ACT" moodCode="EVN">
<!-- Reason for substitution -->
</act> </entryRelationship></act> |
|
|---|
|
UV Substitution Permission
UV Use Period
| Id | 2.16.840.1.113883.10.21.9.1 | Effective Date | 2023‑01‑30 09:55:27Other versions this id:  Useperiod as of 2017‑05‑02 Useperiod as of 2017‑05‑02 Useperiod as of 2017‑01‑02 Useperiod as of 2017‑01‑02
|
|---|
| Status |  Draft Draft | Version Label | 2023 |
|---|
| Name | Useperiod | Display Name | UV Use Period |
|---|
| Description | This element encodes the start and stop time of the medication regimen. This is an interval of time (xsi:type='IVL_TS'), and must be specified as shown. This is an additional constraint placed upon CDA Release 2.0 by this profile, and simplifies the exchange of start/stop and frequency information between EMR systems. |
|
| Classification | CDA Entry Level Template |
|---|
| Open/Closed | Open (other than defined elements are allowed) |
|---|
|
Appendix (Informative)
Acronyms and abbreviations
- C-CDA: Consolidated CDA
- CDA: Clinical Document Architecture
- DSTU: Draft Standard for Trial Use
- EDQM: European Directorate for the Quality of Medicines & Healthcare
- EHR: Electronic Healthcare Record
- HL7: Health Level Seven
- HP: Healthcare Professional
- IDMP: IDentification of Medicinal Products (ISO Standard)
- IHE: Integrating the Healthcare Enterprise
- ISO: International Organization for Standardization
- JIC: Joint Initiative Council on SDO Global Health Informatics Standardization
- LOINC: Logical Observation Identifiers Names & Codes
- MPID: Medicinal Product Identifier
- PCID : Medicinal Product Package Identifier
- PhPID(s): Pharmaceutical Product Identifier(s)
- SDO: Standard Developing Organization
- STU: Standard for Trial Use
- UCUM: Unified Code for Units of Measure
Glossary
- Prescribing is an activity that can be performed by a variety of healthcare professionals and involves a variety of orderable items (see glossary entry). For the purposes of the following Implementation Guide, prescribing is defined as the act of prescribing a medication in either an ambulatory or an institutional setting. This could include initiating a new medication order or making all kinds of modifications to existing orders.
- Dispensing is an activity undertaken to fulfill the logistical requirements of a prescription. It supplies the materials needed to perform the prescribed actions by those who will perform them. Examples of dispensing include eyeglasses, contact lenses and medications. For the purposes of the following ballot material, dispensing is defined as supplying a medication in fulfillment of a prescription or medication order. While dispensing in these circumstances would usually be performed by a pharmacist, other health care providers such as nurses or physicians might also dispense medications.
- Administration is an activity undertaken to give medication to the patient. In the community, this process is usually not recorded, since the majority occurs in the patient's home; only administrations undertaken by a healthcare professional, such as vaccination, tend to be formally documented. Administration of medication in the institutional setting is usually recorded on a dose-by-dose basis, and may be messaged on that basis, or a summary of all the administrations occurring during an inpatient stay may be described.
Integrated examples
The Medication on CDA specification releases are published at the Pharmacy Templates Material Publication Page on HL7 GitHub[4]. This GitHub offers XML materials (also compacted as a ZIP to download) like the W3C schemas and example CDA document instances. A set of use cases have been defined and represented in Medication on CDA format.
Validation artifacts
You can test your implementation (instances) against the Medication on CDA specification. To download materials to your computer for local testing and validation consider...
- ...the W3C schemas (actually valid for any CDA specification) located at the PHARM Materials Page on HL7 GitHub[4].
- ..the ISO schematron, automatically generated by ART-DECOR based on the definitions, located at the Pharmacy Templates Material Publication Page on ART-DECOR[5]. These are files to do validation locally by associating PHARM CDA instances with the main schematron using an XML editor or to use the derived XSLT conversions and apply the according XSLT derivation to your local PHARM CDA instance.
For further information you can follow the documentation.
Operational information
- The original specification is hosted on the logical ART-DECOR main server art-decor.org under the Governance Group HL7 International, the project is reachable at the Project Live Landing Page[6].
Licenses
Following is a non-exhaustive list of third-party terminologies that may require a separate license:
- SNOMED CT: SNOMED International (formerly know as International Healthcare Terminology Standards Development Organization IHTSDO)[7] or info@ihtsdo.org
- Logical Observation Identifiers Names & Codes (LOINC): The Regenstrief Institute, Inc.
- Unified Code for Units of Measure (UCUM) : Regenstrief Institute, Inc. and the UCUM Organization
List of all artifacts used in this guide
CDA Templates
Unconstrained Templates from the original CDA specification
Value Sets
Medication Time Units (UCUM)
| This terminology is a snapshot as of . Terminologies may evolve over time. If you need recent (dynamic) versions of this terminology, please retrieve it from the source. |
| Id | 2.16.840.1.113883.11.21.1 | Effective Date | 2023‑02‑01 11:12:08 |
|---|
| Status |  Final Final | Version Label | 3.0 |
|---|
| Name | MedicationTimeUnits | Display Name | Medication Time Units (UCUM) |
|---|
| Description | Medication Time Units, expressed in UCUM |
|---|
| Usage: 4 | | Id | Name | Type |
|---|
| Template |
|---|
| 2.16.840.1.113883.10.21.9.1 | UV Use Period | DYNAMIC | | 2.16.840.1.113883.10.21.9.1 | UV Use Period (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.21.9.1 | UV Use Period (2023) | DYNAMIC | | 2.16.840.1.113883.10.21.9.1 | UV Use Period (2023) | DYNAMIC |
|
|
| Source Code System | |
|---|
| Level/ Type | Code | Display Name | Code System |
|---|
| 0‑L | a | Year | Unified Code for Units of Measure | | 0‑L | d | Day | Unified Code for Units of Measure | | 0‑L | h | Hour | Unified Code for Units of Measure | | 0‑L | min | Minute | Unified Code for Units of Measure | | 0‑L | mo | Month | Unified Code for Units of Measure | | 0‑L | s | Second | Unified Code for Units of Measure | | 0‑L | wk | Week | Unified Code for Units of Measure |
|
|
| Legenda: Type L=leaf, S=specializable, A=abstract, D=deprecated. NullFlavor OTH (other) suggests text in originalText. HL7 V3: NullFlavors to appear in @nullFlavor attribute instead of @code. |
ActStatusCodeActiveCompletedAbortedSuspended
| This terminology is a snapshot as of . Terminologies may evolve over time. If you need recent (dynamic) versions of this terminology, please retrieve it from the source. |
| Id | 2.16.840.1.113883.11.21.2 | Effective Date | 2017‑03‑06 |
|---|
| Status |  Draft Draft | Version Label | |
|---|
| Name | ActStatusCodeActiveCompletedAbortedSuspended | Display Name | ActStatusActiveCompletedAbortedSuspended |
|---|
| Usage: 6 | | Id | Name | Type |
|---|
| Template |
|---|
| 2.16.840.1.113883.10.21.4.1 | UV Medication Order (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.21.4.6 | UV Subordinate Substance Administration (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.21.4.1 | UV Medication Order (2023) | DYNAMIC | | 2.16.840.1.113883.10.21.4.1 | UV Medication Order (STU1) | DYNAMIC | | 2.16.840.1.113883.10.21.4.1 | UV Medication Order (2023) | DYNAMIC | | 2.16.840.1.113883.10.21.4.6 | UV Subordinate Substance Administration (2023) | DYNAMIC |
|
|
| Source Code System | |
|---|
| Level/ Type | Code | Display Name | Code System |
|---|
| 0‑L | completed | Completed | ActStatus | | 0‑L | aborted | Aborted | ActStatus | | 0‑L | active | Active | ActStatus | | 0‑L | suspended | Suspended | ActStatus |
|
|
| Legenda: Type L=leaf, S=specializable, A=abstract, D=deprecated. NullFlavor OTH (other) suggests text in originalText. HL7 V3: NullFlavors to appear in @nullFlavor attribute instead of @code. |
Mood Code Evn Int Rqo
| This terminology is a snapshot as of . Terminologies may evolve over time. If you need recent (dynamic) versions of this terminology, please retrieve it from the source. |
| Id | 2.16.840.1.113883.11.21.4 | Effective Date | 2018‑03‑21 |
|---|
| Status |  Draft Draft | Version Label | |
|---|
| Name | MoodCodeEvnIntRqo | Display Name | Mood Code Evn Int Rqo |
|---|
| Usage: 2 | | Id | Name | Type |
|---|
| Template |
|---|
| 2.16.840.1.113883.10.21.4.6 | UV Subordinate Substance Administration (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.21.4.6 | UV Subordinate Substance Administration (2023) | DYNAMIC |
|
|
| Source Code System | |
|---|
| Level/ Type | Code | Display Name | Code System |
|---|
| 0‑L | EVN | Event | Act Mood | | 0‑L | INT | Intent | Act Mood | | 0‑L | RQO | Request | Act Mood |
|
|
| Legenda: Type L=leaf, S=specializable, A=abstract, D=deprecated. NullFlavor OTH (other) suggests text in originalText. HL7 V3: NullFlavors to appear in @nullFlavor attribute instead of @code. |
Unknown or absent medication
| This terminology is a snapshot as of . Terminologies may evolve over time. If you need recent (dynamic) versions of this terminology, please retrieve it from the source. |
| Id | 2.16.840.1.113883.11.21.5 | Effective Date | 2018‑03‑21 |
|---|
| Status |  Draft Draft | Version Label | |
|---|
| Name | Unknownorabsentmedication | Display Name | Unknown or absent medication |
|---|
| Copyright | This artefact includes content from SNOMED Clinical Terms® (SNOMED CT®) which is copyright of the International Health Terminology Standards Development Organisation (IHTSDO). Implementers of these artefacts must have the appropriate SNOMED CT Affiliate license - for more information contact http://www.snomed.org/snomed-ct/getsnomed-ct or info@snomed.org. |
|---|
| Usage: 5 | | Id | Name | Type |
|---|
| Template |
|---|
| 2.16.840.1.113883.10.21.4.7 | UV Medication Statement (STU1) | DYNAMIC | | 2.16.840.1.113883.10.21.4.7 | UV Medication Statement (2023) | DYNAMIC | | 2.16.840.1.113883.10.21.4.13 | UV Medication Administration (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.21.4.7 | UV Medication Statement (R1-STU2-ballot) | DYNAMIC | | 2.16.840.1.113883.10.21.4.13 | UV Medication Administration (2023) | DYNAMIC |
|
|
| Source Code System | |
|---|
| Level/ Type | Code | Display Name | Code System |
|---|
| 0‑L | 182904002 | Drug treatment unknown (finding) | SNOMED Clinical Terms | | 0‑L | 182849000 | No drug therapy prescribed (situation) | SNOMED Clinical Terms |
|
|
| Legenda: Type L=leaf, S=specializable, A=abstract, D=deprecated. NullFlavor OTH (other) suggests text in originalText. HL7 V3: NullFlavors to appear in @nullFlavor attribute instead of @code. |
Referenced HL7 Version 3 Value Sets
- 2.16.840.1.113883.1.11.13955 ActEncounterCode
- 2.16.840.1.113883.1.11.16208 ActPharmacySupplyType
- 2.16.840.1.113883.1.11.16866 ActPriority
- 2.16.840.1.113883.1.11.15933 ActStatus
- 2.16.840.1.113883.1.11.19708 ActSubstanceAdministrationCode
- 2.16.840.1.113883.1.11.16621 ActSubstanceAdminSubstitutionCode
- 2.16.840.1.113883.1.11.14570 AdministrableDrugForm
- 2.16.840.1.113883.1.11.11526 HumanLanguage
- 2.16.840.1.113883.11.20.9.18 MoodCodeEvnInt
- 2.16.840.1.113883.1.11.78 Observation Interpretation
- 2.16.840.1.113883.1.11.14079 ObservationMethod
- 2.16.840.1.113883.1.11.14581 RouteOfAdministration
- 2.16.840.1.113883.1.11.19719 SubstanceAdminSubstitutionNotAllowedReason
- 2.16.840.1.113883.1.11.19377 SubstanceAdminSubstitutionReason
- 2.16.840.1.113883.1.11.10706 TimingEvent
- 2.16.840.1.113883.1.11.19447 x_ActRelationshipEntryRelationship
- 2.16.840.1.113883.1.11.19890 x_ActStatusActiveComplete
Datatypes
Datatypes for element definitions used
- ANY – ANY
- BL – Boolean
- CD – Concept Descriptor
- CE – Coded with Equivalents
- CS – Coded Simple Value
- ED – Encapsulated Data
- EN – Entity Name
- II – Instance Identifier
- INT – Integer
- INT.NONNEG – Interval of Integer, non-negative
- IVL_INT – Interval of Integer
- IVL_PQ – Interval of Physical Quantity
- IVL_TS – Interval of Time Stamp
- IVXB_TS – Interval Boundary of Time Stamp
- PIVL_TS – Periodic Interval of Timezone
- PQ – Physical Quantity
- RTO_PQ_PQ – Ratio Physical Quantity / Physical Quantity
- ST – Character String
- TEL – Telecommunication Address
- TS – Time Stamp
Datatypes for attributes used
- bl – boolean code
- cs – code
- uid – identifier
Extensions
Detailed medications information
This specification uses CDA extensions in order to provide details about medications, as further described in the section on the design conventions for Medicinal Product Identification and as used in template 2.16.840.1.113883.10.21.4.11 UV Medication Information (detail). The extension uses the namespace
urn:hl7-org:pharm
This is the list of elements defined for that template.
- pharm:formCode (Administrable Pharmaceutical Dose Form)
- pharm:asContent (Packaging of the medication)
- pharm:quantity
- pharm:containerPackagedMedicine (Most inner Package Item or the Packaged Medicinal Product)
- pharm:code
- pharm:name (Name of the Package Item or of the Packaged Medicinal Product)
- pharm:formCode (type of the most inner package item or of the or the Packaged Medicinal Product)
- pharm:capacityQuantity (the functional capacity of the container)
- pharm:asContent (Containing package)
- pharm:quantity
- pharm:containerPackagedMedicine (Intermediate Package Item or the Packaged Medicinal Product)
- pharm:code
- pharm:name (Name of the Package Item or of the Packaged Medicinal Product)
- pharm:formCode (type of the intermediate package item or of the or the Packaged Medicinal Product)
- pharm:capacityQuantity (the functional capacity of the container)
- pharm:asContent (Containing package)
- pharm:quantity
- pharm:containerPackagedMedicine (Packaged Medicinal Product)
- pharm:code
- pharm:name (Name of the Packaged Medicinal Product)
- pharm:formCode (type of the Packaged Medicinal Product)
- pharm:capacityQuantity (the functional capacity of the container)
- pharm:asSpecializedKind (used to represent any classification of the product (ATC code, future PhPIDs,..) )
- pharm:generalizedMaterialKind
- pharm:ingredient (list of active substances used for this product)
- pharm:quantity (strength)
- pharm:ingredientSubstance (active substance)
How to read the table view for templates
The template definitions are shown in a table view. It is comprised of Template Meta data and the Template Design. For further information please refer to the HL7 Templates Standard: Specification and Use of Reusable Information Constraint Templates, Release 1[2].
Templates may also be included in the hierarchical graph view (often used for CDA), see below.
Template Meta data
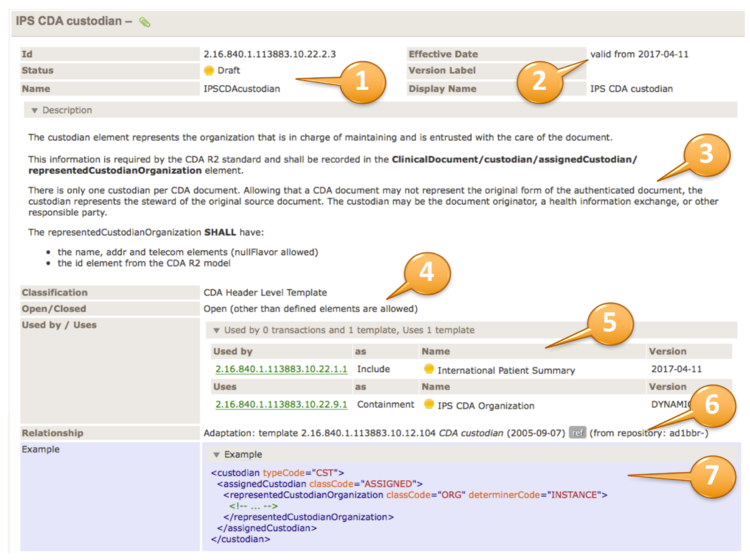
The upper right part of the template table contains the template meta data. Template id, status and the template name are shown (1). Furthermore the Version (effective date), a possible version label and the display name are shown (2).
The description area (plain or an accordion) contains the template descriptions/purpose (3), followed by classifications and whether the template is defined as open or closed (4).
The usage part (5) may list templates that uses this template or what templates this templates uses. A relationship list (6) may show all relationships to other templates or models.
Examples may show the correct use of the template by an XML fragment (7).
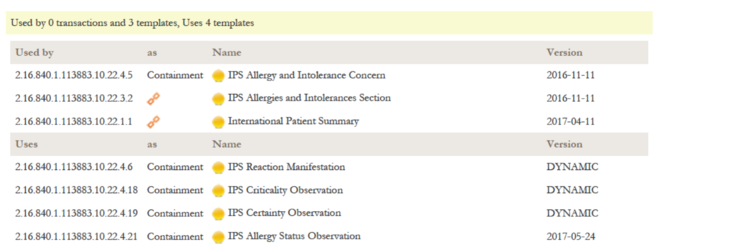
The relationship list shows all relationships to other templates or models for this template. It is divided in the "Used by" part listing templates that make use of this template, and a "Uses" listing all templates that are used by this templates, either as inclusion or containment. Indirect relationships like the parent Document Level Template for a Section Level Template are marked with a chain symbol.
The PDF version is rendered in the same way, but maybe with different fonts etc. to fit customized publication requirements.
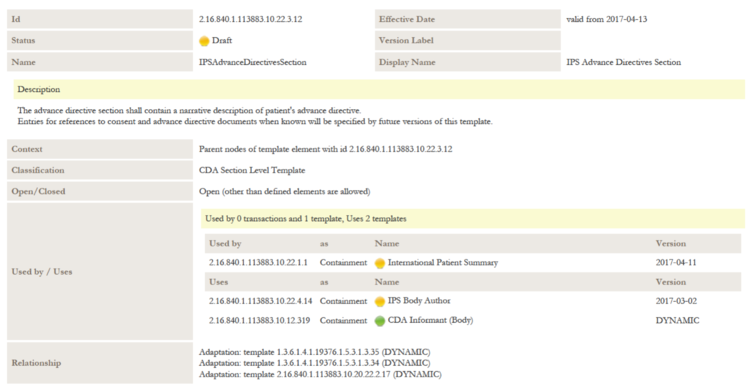
Table view of Template Design
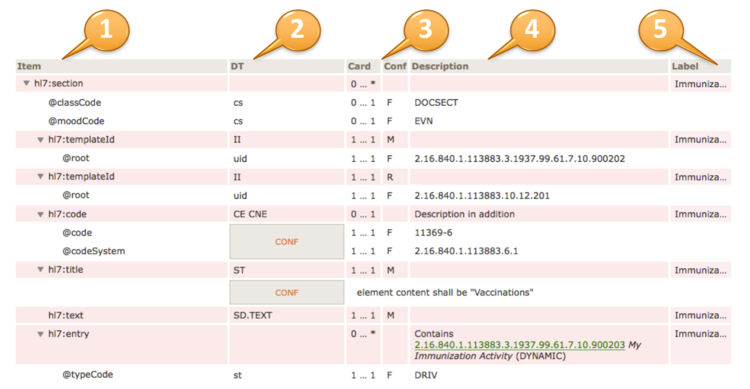
The headings of the table view of a template design are:
Item (1) contains the XML document tree view of all elements and attributes specified in the template design. Elements are denoted by a preceding triangle and attributes by a preceding "@".
DT (2) data types, contains the data type of the item, for more information on valid data types for element and attributes (see [2]).
Card / Conf (3) cardinality (Card) and conformance (Conf) of the item.
Cardinality is the usual notion of min and max occurrences of the element. For attributes 0..1 denotes optionality, 1..1 say that the attribute is required and NP denotes prohibited attributes.
Conformance may display values as shown in the following table.
Values of the conformance column
| Conf |
Short |
Description
|
| O |
optional |
Data is truly optional
|
| R |
required |
If data is present and not masked (e.g. for privacy reasons), it must be provided, otherwise it may be omitted or explicitly null flavored.
Sender and receiver must support this element.
|
| M |
mandatory |
The data must be populated with a valid value from the associated value domain, otherwise the instance is not valid and may not be communicated.
Sender and receiver must support this element.
|
| C |
conditional |
There are conditions when data has to be provided (e.g. co-constraints like "information about pregnancy IF the patient is "female".
Sender and receiver must support this element.
|
| F |
fixed |
The data has a fixed value.
|
| NP |
not permitted |
Data shall not be present
|
Description (4) contains a textual description of the item, may also contain constraints and values for fixed attributes.
Label (5) is a human readable label that is displayed upon errors, warnings or notes during validation.
Details of the table view
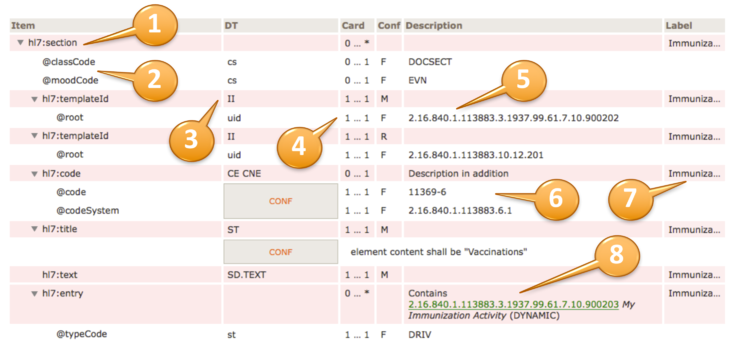
The actual template design shows the XML structure in a hierarchical list of elements (items) that are typically prefixed by the namespace "hl7:" or "cda:" (1).
Elements are denoted with a triangle, attributes with an @ sign (2).
Data types are specified according to the list of supported data types (3). They may be simple data types (lowercase), regular data types (uppercase) or flavors thereof.
In case of coded elements, the coding strength (Required/CNE, Extensible/CWE, Preferred or Example) can be highlighted near the datatype (e.g. “CD.IPS (Extensible/CWE)”) ; the absence of indications about the strength (e.g. “CE.IPS”) shall be interpreted as “Required/CNE”.
Values of the coding strength column
| Strength |
Displayed as |
Description
|
| Required |
Required/CNE |
Coded with no exceptions; this element SHALL be from the specified value set
|
| Extensible |
Extensible/CWE |
Coded with Exceptions; this element SHALL be from the specified value set if any of the codes within the value set can apply to the concept being communicated. If the value set does not cover the concept (based on human review), alternate codings (or, data type allowing, text) may be included instead.
|
| Preferred |
Preferred |
Instances are encouraged to draw from the specified codes for interoperability purposes but are not required to do so to be considered conformant.
|
| Example |
Example |
Instances are not expected or even encouraged to draw from the specified value set. The value set merely provides examples of the types of concepts intended to be included.
|
The cardinality and conformance column is explained above (4).
Fixed values for e.g. attributes are also shown in the "description" column (5), preceded by a "F" in the Conf column.
Conformance statements are shown together with a CONF box, e.g. a @code and a @codeSystem with fixed and required values (6).
An optional label is displayed at the rightmost column (7).
Inclusion or containments of other templates, e.g. an entry within a section, are shown accordingly (8) along with their template id, display name and flexibility/stability indication, i.e. "DYNAMIC" (the most recent version) or a STATIC binding together with a version date.

Choices of elements are shown as a choice list with the elements in questions summarised in a bullet point list.

A typical Conformance Statement is the binding of a coded element to a value set. This is expressed in the way shown. The value set is represented with the id, display name and the flexibility/stability of the binding.

In case a constraint is expressed in words, a box "Constraint" accompanies the textual expression of the constraint.

In cases where constraints are expressed by formalised rules in ISO Schematron, the rule along with the role (error, warning), the test and the assertion message is shown.
How to read the Templates hierarchical graph view

Templates are often included in the hierarchical graph view (often used for CDA). It gives an overview of e.g. section and entries and their nesting/relationships.

In case a template has more that one type (CDA Person for header, section and entry templates), it is denoted with a *, if a recursive definition is detected, this is shown with the symbol @.
How to read the where criteria
Templates sometimes include criteria for identifying distinct elements from a list (e.g. in a choice).
The criteria used to identify the items are shown in square brackets using the assertion where [ criteria ]
Criteria can be:
- an xpath expression as in the example : where [hl7:low or hl7:high]
- or an integer indexing the items of the list: e.g. where [1]; where [2]
References
Literature
- Boone KW: The CDA Book. Springer 2011, ISBN 978-0-85729-336-7
Links
- ↑ 1.0 1.1 http://www.hl7.org/implement/standards/product_brief.cfm?product_id=379
- ↑ 2.0 2.1 2.2 2.3 HL7 Templates Standard: Specification and Use of Reusable Information Constraint Templates, Release 1 http://www.hl7.org/implement/standards/product_brief.cfm?product_id=377
- ↑ ISO/TS 13582:2013 Health informatics -- Sharing of OID registry information
- ↑ 4.0 4.1 Pharmacy Templates Material Publication Page on HL7 GitHub https://github.com/HL7/CDA-pharma
- ↑ Pharmacy Templates Material Publication Page on ART-DECOR https://hl7intl.art-decor.pub/index.php?prefix=pharmcda-
- ↑ Pharmacy Templates Project Live Landing Page https://art-decor.org/ad/#/pharmcda-/project/overview
- ↑ Get SNOMED CT http://www.ihtsdo.org/snomed-ct/get-snomed-ct
Figures
- ↑ Locating ballot comments
![]() Draft or
Draft or ![]() Pending are subject to ballot comments.
Pending are subject to ballot comments.
![]() Final or Active are not (directly) part of the ballot and some artifacts actually even come from external sources. Reference artifacts are indicated by the symbol
Final or Active are not (directly) part of the ballot and some artifacts actually even come from external sources. Reference artifacts are indicated by the symbol