FR2
Functional requirements and high-level use cases
The International Patient Summary, i.e. a “specialty-agnostic, condition-independent" summary, was initially designed for cross-border unscheduled care, but its actual adoption is not limited to this usage scenario.
Patient summary initiatives are currently in various stages of development in different parts of the world. A summary of the most recent initiatives and experiences across the world can be found in the https://international-patient-summary.net/implementations-across-the-globe/.
As mentioned there are several options in terms of creation, sharing and usage of an IPS. For example:
- An IPS can be created when requested and used before, during, or after a care episode; or can be asynchronously generated and made available for future usage (e.g. store and retrieve).
- The IPS can be retrieved using a document exchange infrastructure; transported by the patient; or shared using cloud-services.
- The IPS may be subject of a transformation process that may include syntactical conversions, coded concept mappings and coded concept designation or free text translations. This transformation process may be performed in the creation phase, during the transmission, or after the IPS has been received, possibly using an external service.
- Finally, the received CDA may be used in different ways. For example, displayed using a common CDA stylesheet; display the extracted relevant information; incorporated into the receiver’s EHR. Alternatively, a specialized viewer may be adopted to enable the display of the translated content.
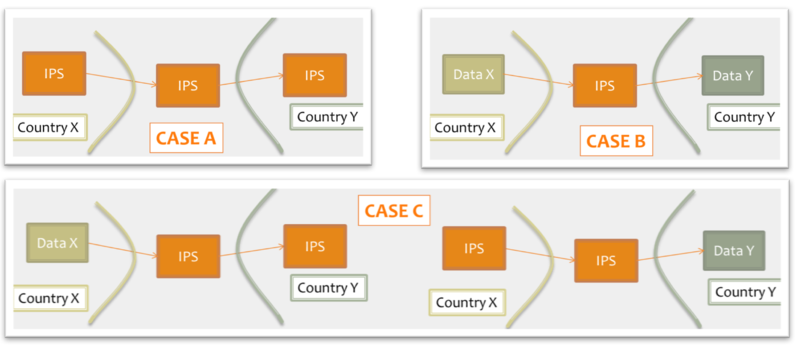
Moreover, for cross-jurisdiction exchange, the IPS could be used as:
- shared format among jurisdictions (case A), where jurisdictions originate and use IPS conforming documents unaltered.
- pivot document among existing summaries / data formats (case B). For example, the IPS is used as intermediate format between the US C-CDA CCD (please note that the CCD scope differs from that of the IPS) and the European eHDSI Patient Summary for a Transatlantic Patient Summary exchange.
- mixed mode (Case C), where either the originator or the consumer is expected to use an IPS conforming document.
Figure: Examples of IPS usage
An International Patient Summary may:
- be the result of automatic assembly (assembled IPS) or of a human summarization act (human curated IPS)
- have one or more EHR sources
- document information from a single or multiple jurisdictions/organizations
- aggregate input from a single or multiple encounters.
A clear determination of such contextual information raises the trustworthiness of the received IPS and helps the appropriate usage of its content by the receiver .
Most of these aspects are related to data provenance introduced in Chapter 5: Provenance.
Finally, there are several technical infrastructures and services that may be designed to support these requirements.
That said, it is out of scope of this standard to:
- give indications about solutions and strategies for the IPS creation, sharing, syntactical and semantic mapping, translation, and use.
- Provide guidance on how to determine the relevance of data for their inclusion in a IPS.
- Define selection or composition rules for facing potential inconsistencies from multiple sources in case of automatic collection.
- Impose constraints on the possible active Patient Summaries. Many jurisdictions require that only one active Patient Summary for unscheduled care is made available, but this is an implementation choice.