Difference between revisions of "IPS Design conventions and principles 1"
(→Unmapped Coded Concepts) |
(→The use of medication statements in the summary) |
||
| (23 intermediate revisions by 3 users not shown) | |||
| Line 31: | Line 31: | ||
Other kinds of negations such as: (a) the negation of an allergy to a specific agent; (b) the absence of a particular disease; or (c) the fact that a specific vaccination has not been performed, have been considered beyond the set of essential data for an IPS. | Other kinds of negations such as: (a) the negation of an allergy to a specific agent; (b) the absence of a particular disease; or (c) the fact that a specific vaccination has not been performed, have been considered beyond the set of essential data for an IPS. | ||
| − | This specification represents this core set of negations (“general condition/activity unknown” and “general condition/activity/known absent” ) | + | This specification represents this core set of negations (“general condition/activity unknown” and “general condition/activity/known absent” ) using explicit coded elements rather than relying on specific mechanisms of HL7 CDA such as nullFlavor and negationInd attributes or human readable text (possibly not understood by the foreign country receiver). |
| + | |||
| + | In contrast to the practice to use negationInd or nullFlavor attributes on a section itself, we prohibit the use of these attributes on section level to express “unknown” or “no information” situations. A section holds the categorized (coded) narrative part of the documented activity and will never carry negationInd or nullFlavor attributes. Instead, we enforce by design, that “unknown” or “no information” expressions always go to the coded entry with a corresponding act code. | ||
The main reasons for this choice are: | The main reasons for this choice are: | ||
| Line 37: | Line 39: | ||
* To make clinical content representation less dependent on a particular format or syntax, enabling a more practical path to transforming and exchanging data from one standard format (e.g. CDA R2) to another (e.g. FHIR). | * To make clinical content representation less dependent on a particular format or syntax, enabling a more practical path to transforming and exchanging data from one standard format (e.g. CDA R2) to another (e.g. FHIR). | ||
* to provide one single method to express the presence or absence of a particular condition (e.g. an allergy) or activity (e.g. an immunization), or the lack of knowledge regarding this kind of condition or activity, resulting in a more robust and easily implementable specification. | * to provide one single method to express the presence or absence of a particular condition (e.g. an allergy) or activity (e.g. an immunization), or the lack of knowledge regarding this kind of condition or activity, resulting in a more robust and easily implementable specification. | ||
| − | |||
| − | |||
For the other kinds of negations, not explicitly mentioned in this guide, it is suggested to apply – where possible – the same approach. | For the other kinds of negations, not explicitly mentioned in this guide, it is suggested to apply – where possible – the same approach. | ||
| Line 81: | Line 81: | ||
== Unmapped Coded Concepts == | == Unmapped Coded Concepts == | ||
| − | In several real world situations the records used as source for the Patient Summaries may use locally adopted terminologies mapped into the reference value sets. When the original coded concept cannot be mapped in one of the coded concepts included in the reference value sets, it is recommended | + | In several real world situations the records used as source for the Patient Summaries may use locally adopted terminologies mapped into the reference value sets. When the original coded concept cannot be mapped in one of the coded concepts included in the reference value sets, it is recommended to populate the original code in the IPS instance (in a 'translation' sub-element), with a nullFlavor indicating that the mapping didn’t occur. (See also the [[#Concept code mapping| Concept code mapping]] in the functional requirements section.). The nullFlavor value depends upon the coding strength of the binding. |
Two circumstances are considered here: the case in which the coding strength is Required (CNE) and when it is Extensible (CWE). | Two circumstances are considered here: the case in which the coding strength is Required (CNE) and when it is Extensible (CWE). | ||
| Line 127: | Line 127: | ||
Functional requirements exposed in [[#Concept code mapping|section 3.1]] (multi-coding, link to original text, mapping between codes of the same code system) are also detailed below. | Functional requirements exposed in [[#Concept code mapping|section 3.1]] (multi-coding, link to original text, mapping between codes of the same code system) are also detailed below. | ||
| − | Case 1: | + | Case 1: Single local code mapped to primary code from the reference value set. |
<syntaxhighlight lang="xml"> | <syntaxhighlight lang="xml"> | ||
| Line 144: | Line 144: | ||
''The square brackets [ ] are used to indicate that the originalText element may or may not be present'' | ''The square brackets [ ] are used to indicate that the originalText element may or may not be present'' | ||
| − | Case 2: | + | Case 2: Multiple local codes mapped together using nested 'translation' elements, and mapped to the primary code from the reference value set. |
<syntaxhighlight lang="xml"> | <syntaxhighlight lang="xml"> | ||
| Line 168: | Line 168: | ||
</syntaxhighlight> | </syntaxhighlight> | ||
| − | Case 3: | + | Case 3: Original and the reference coded concepts belong to the same code system (distinct codes). This may be the result of a different level of granularity between the original term and the reference value sets. |
<syntaxhighlight lang="xml"> | <syntaxhighlight lang="xml"> | ||
| Line 178: | Line 178: | ||
</syntaxhighlight> | </syntaxhighlight> | ||
| − | ''Note: The R1 data type definition identifies the <translation> as “a set of other concept descriptors that translate this concept descriptor into other code systems. There is however a common understanding that | + | ''Note: The R1 data type definition identifies the <translation> as “a set of other concept descriptors that translate this concept descriptor into other code systems." There is however a common understanding that it may be more than one representation in a single code system where code systems allow multiple representations, such as SNOMED CT. Data Types R2 extended the possibility to provide translations also in the same code system.'' |
== Translation of designations == | == Translation of designations == | ||
| Line 184: | Line 184: | ||
The capability of recording one or more designations, in different languages, for the exchanged Patent Summary is one of the functional requirements requested for “safety and liability reasons” by the European Cross-border services (see [[#Designations’ Translation| Designations’ Translation]] under the [[#Functional requirements and high-level use cases | Functional requirements and high-level use cases ]] for more details). | The capability of recording one or more designations, in different languages, for the exchanged Patent Summary is one of the functional requirements requested for “safety and liability reasons” by the European Cross-border services (see [[#Designations’ Translation| Designations’ Translation]] under the [[#Functional requirements and high-level use cases | Functional requirements and high-level use cases ]] for more details). | ||
| − | Given that the base | + | Given that the base CDA R2 standard which uses datatypes R1 does not offer a native way to convey the language translation of 'displayName', this guide introduces an optional 'ips:designation' extension to the CD datatype for that purpose. |
Below are examples of usage of this extension. | Below are examples of usage of this extension. | ||
| Line 192: | Line 192: | ||
<code code="60591-5" codeSystem="2.16.840.1.113883.6.1" | <code code="60591-5" codeSystem="2.16.840.1.113883.6.1" | ||
codeSystemName="LOINC" displayName="Patient Summary"> | codeSystemName="LOINC" displayName="Patient Summary"> | ||
| − | <ips:designation | + | <ips:designation language="it-IT">Profilo Sanitario Sintetico</ips:designation> |
| − | <ips:designation | + | <ips:designation language="fr-FR">Patient Summary</ips:designation> |
| − | <ips:designation | + | <ips:designation language="en">Patient Summary</ips:designation> |
</code> | </code> | ||
</syntaxhighlight> | </syntaxhighlight> | ||
| − | '' | + | ''Including code mapping'' |
<syntaxhighlight lang="xml"> | <syntaxhighlight lang="xml"> | ||
<value xsi:type="CD" code="42338000" codeSystem="2.16.840.1.113883.6.96" | <value xsi:type="CD" code="42338000" codeSystem="2.16.840.1.113883.6.96" | ||
displayName="Salmonella-gastroenterit"> | displayName="Salmonella-gastroenterit"> | ||
| − | <ips:designation | + | <ips:designation language="da-DK">Salmonella-gastroenterit</ips:designation> |
| − | <ips:designation | + | <ips:designation language="en">Salmonella gastroenteritis (disorder)</ips:designation> |
[ | [ | ||
<originalText> | <originalText> | ||
| Line 216: | Line 216: | ||
== Narrative Translations == | == Narrative Translations == | ||
| − | + | “Narrative translation” means both the translation of the original narrative text, that can be human curated or automatically performed, and the generation of a translated narrative based on the coded entries. | |
| − | The functional requirements associated | + | The functional requirements associated with this process are described in the [[#Designations’ Translation| Designations’ Translation]] section under [[#Functional requirements and high-level use cases | Functional requirements and high-level use cases]], and can be summarized in two main points : (a) language identification and (b) distinguishable original and translated narratives. |
Moreover, the methodology applied for the narrative translation (e.g. derived from the coded entries; translated by a generic service;..) should be noted. | Moreover, the methodology applied for the narrative translation (e.g. derived from the coded entries; translated by a generic service;..) should be noted. | ||
| − | Regarding translation of section narrative <text>, this guide recommends | + | Regarding the translation of section narrative <text>, this guide recommends providing this translation on purely textual subordinate sections (one per translation) as specified in the IPS Translation Section (2.16.840.1.113883.10.22.3.15) template. |
| − | + | An example of this is: | |
<syntaxhighlight lang="xml"> | <syntaxhighlight lang="xml"> | ||
| Line 276: | Line 276: | ||
Each Observation contained by a Concern Act is a discrete observation of a condition. Its statusCode is always “completed” since it is the “status of the activity of observing, not the status of what is being observed”. | Each Observation contained by a Concern Act is a discrete observation of a condition. Its statusCode is always “completed” since it is the “status of the activity of observing, not the status of what is being observed”. | ||
| − | The clinical status of a condition (e.g. a disease, an allergy,..) is instead recorded by | + | The clinical status of a condition (e.g. a disease, an allergy,..) is instead recorded by specialized subordinated observations (IPS Allergy Status Observation; IPS Problem Status Observation), documenting whether it is active, in remission, resolved, et cetera. |
The effectiveTime, also referred to as the "biologically relevant time", is the time at which the observation holds for the patient. For a provider seeing a patient in the clinic today, observing a history of penicillin allergy that developed six months ago, the effectiveTime is six months ago. | The effectiveTime, also referred to as the "biologically relevant time", is the time at which the observation holds for the patient. For a provider seeing a patient in the clinic today, observing a history of penicillin allergy that developed six months ago, the effectiveTime is six months ago. | ||
The effectiveTime of the Observation gives an indication of whether or not the underlying condition is resolved, but the current status is documented by a subordinated observation. | The effectiveTime of the Observation gives an indication of whether or not the underlying condition is resolved, but the current status is documented by a subordinated observation. | ||
| Line 289: | Line 289: | ||
The confirmation status, also noted as verification status or certainty, indicates the certainty associated with a condition (e.g. confirmed, provisional, refuted,…) providing information about the potential risk, for example, of a reaction to the identified substance. | The confirmation status, also noted as verification status or certainty, indicates the certainty associated with a condition (e.g. confirmed, provisional, refuted,…) providing information about the potential risk, for example, of a reaction to the identified substance. | ||
| − | The confirmation status of a condition (e.g. a disease, an allergy,..) is recorded by a | + | The confirmation status of a condition (e.g. a disease, an allergy,..) is recorded by a specialized subordinated observation (IPS certainty Observation), documenting whether the condition is confirmed, unconfirmed, provisional, refuted, et cetera. |
== The use of medication statements in the summary == | == The use of medication statements in the summary == | ||
| − | + | A medication list may strongly vary depending on the context of use (e.g. support for prescription or dispensation, drug reconciliation, etc. ) and on the type of information reported (e.g. patient-reported medication, prescribed, dispensed or administered medications, active or past medications, etc.). | |
| − | |||
| − | |||
| − | + | This is still true also for the medication summary in a Patient Summary. It could be, for instance, a list of "Relevant prescribed medicines whose period of time indicated for the treatment has not yet expired whether it has been dispensed or not" (European guidelines on Patient Summary <ref>EHN Guideline on the electronic exchange of health data under Cross-Border Directive 2011/24/EU. Release 2. (https://ec.europa.eu/cefdigital/wiki/display/EHOPERATIONS/Business+Analysis+and+Requirements+Management?preview=/55878732/55878698/(Adopted)%20Patient%20Summary%20Guideline%20cross-border%20exchange%20of%20health%20data%20(release%202).pdf</ref>); a list of actually dispensed medications; a list of relevant medications for the patient (IHE PCC <ref>IHE Patient Care Coordination Technical Framework http://ihe.net/Technical_Frameworks/#pcc</ref>); or conversely, it could be a complete history including the full patient's prescription and dispensation history and information about intended drug monitoring (C-CDA <ref name="ccda21">HL7 C-CDA Implementation Guide DSTU R2.1 http://www.hl7.org/implement/standards/product_brief.cfm?product_id=379</ref>). | |
| − | This information need, in line with the IPS principle of minimum non exhaustive data, is well expressed by the concept of Medication Statement (see https://www.hl7.org/fhir/medicationstatement.html). | + | Moreover, for the scope of the International Patient Summary, it is important to know what medications are being taken by or have been given to a patient; without necessarily providing all the details about the medication order, supply, administration or monitoring. This information need, in line with the IPS principle of minimum non exhaustive data, is well expressed by the concept of Medication Statement (see https://www.hl7.org/fhir/medicationstatement.html). |
| − | The medication summary is therefore a list of relevant medication statements, built from either a prescription list or a dispensation list.In fact, in many practical cases data included in a Patient Summary are derived from the list of | + | The IPS medication summary is therefore a list of relevant medication statements, possibly built from either a prescription list or a dispensation list. In fact, in many practical cases data included in a Patient Summary are derived from the list of the medicines prescribed by a GP and recorded in its EHR-S; or extracted from a in regional/national prescribing and/or dispensation systems. In these cases, data obtained from actual dispensation and/or prescription records can be still recorded in the IPS as statements and the original request, supply or administration records referred by the statement if really needed. |
==Medicinal Product Identification== | ==Medicinal Product Identification== | ||
| − | The identification of medicinal products is quite easily solved within a single jurisdiction relying on local | + | The identification of medicinal products is quite easily solved within a single jurisdiction relying on local drug databases. In contrast, it is one of the major open issues for eHealth services across jurisdictions. |
| − | The set of ISO standards called IDMP<ref>IDMP standards https://www.idmp1.com/idmp-standards</ref> - designed initially for the regulatory | + | The set of ISO standards called IDMP<ref>IDMP standards https://www.idmp1.com/idmp-standards</ref> - designed initially for the regulatory scope, but hopefully extensible to other domains - are the most promising solution for solving this known issue, as also highlighted by the European project OpenMedicine <ref>European project OpenMedicine http://www.open-medicine.eu/home.html</ref>. The completion of the IDMP implementation guides, the deployment of the needed supporting services, and the development of some companion standards that will allow the seamless flow of the IDMP identifiers and attributes from the regulatory space to the clinical world (and back) are however still in progress. Additional time is needed before these identifiers and attributes will be available in full for practical use. |
| − | Following therefore the IPS principles of “implementability”, “openness and extensibility” the solution proposed here does not rely on IDMP identifiers. Nonetheless, | + | Following therefore the IPS principles of “implementability”, “openness" and "extensibility”, the solution proposed here does not rely on IDMP identifiers. Nonetheless, it takes into account, however, relevant IDMP identifiers and attributes which are already usable in the IPS, namely the Pharmaceutical Product Identifiers (PhPIDs), the Medicinal Product Identifier (MPID), and the Medicinal Product Package Identifier (PCID). |
| − | ''Note: IDMP Medicinal Product (MPID) and Medicinal Product Package (PCID) identifiers | + | ''Note: IDMP Medicinal Product (MPID) and Medicinal Product Package (PCID) identifiers depend on the market authorization. The “same” product might therefore have different IDs if different authorizations have been received in different countries, while the PhPID should be the same. For the purpose of the IPS, future standards and implementation guides should define global product identifiers that do not depend on the drug registration process (as the Virtual Medicinal Product in SNOMED CT) and relate to IDMP identifiers.'' |
| − | Thus, in absence of a global identification system for medicinal products, the solution here | + | Thus, in the absence of a global identification system for medicinal products, the solution proposed here is based on the approach initially adopted by the European cross-border services (epSOS and currently by the eHDSI project), reused by the IHE Pharmacy templates and more recently adopted (for specific cases) by the HL7 Pharmacy Medication statement templates. The main idea is to integrate local drug identifiers (e.g. product codes) with all the relevant identifying and descriptive attributes that may help the receiver to understand the type of product the sender is referring to, e.g.: active ingredients, administrative dose forms, strength, route of administration and package description. |
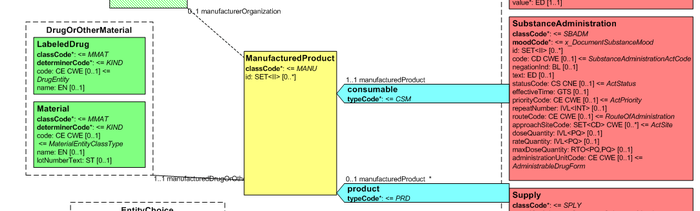
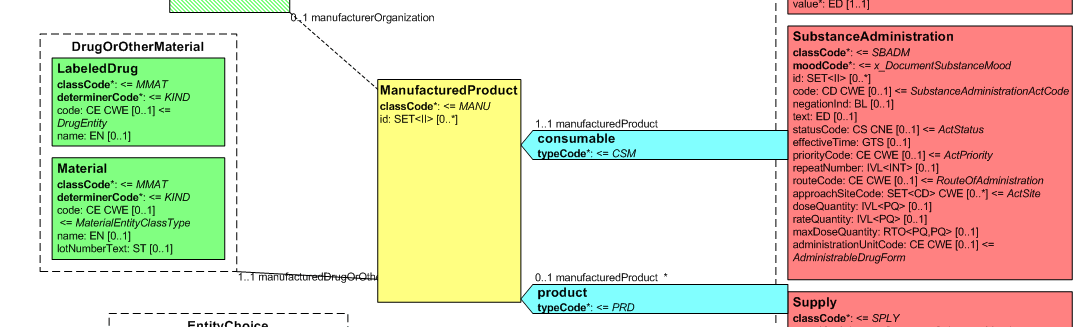
| − | + | Medication data is usually represented in the CDA Templates using the manufacturedMaterial class, which includes a code and a name to describe any level of the product: packaged product, medicinal product, classes or clusters or products, and so on. This information is not however sufficient for covering the requirements of the IPS. | |
{{IncludeImage|IPS_CDA_SBDAM.png|700px|90%}} | {{IncludeImage|IPS_CDA_SBDAM.png|700px|90%}} | ||
| Line 321: | Line 319: | ||
<ref group="Figure">Representation of medicines in CDA</ref> Representation of medicines in CDA | <ref group="Figure">Representation of medicines in CDA</ref> Representation of medicines in CDA | ||
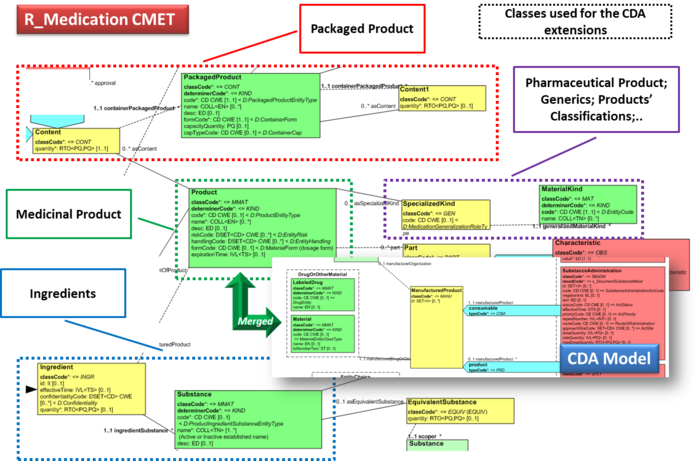
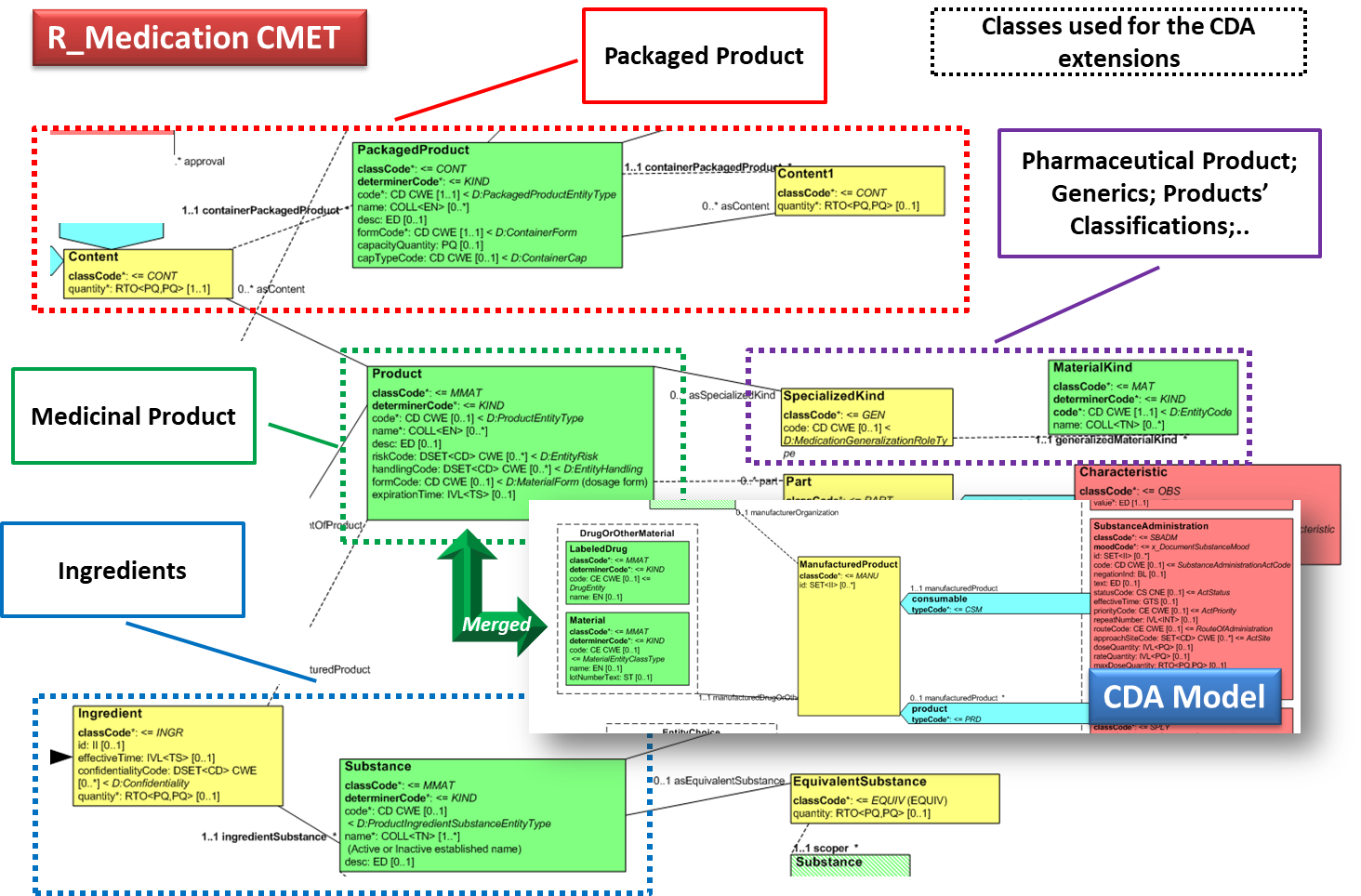
| − | Hence, in order to describe these attributes | + | Hence, in order to describe these attributes the CDA model has been extended enhancing the Manufactured Material class with attributes and relationships derived from the latest published version of the R_Medication CMET (see HL7 V3 Normative Edition 2017) based on the HL7 Common Product Model (''“The common product model is used to improve the alignment between the different representations of products used within the body of HL7 Version 3 models. One goal of this effort is to make it possible to have a single representation, which could then be used as a common message element type (CMET) across those models.”'') . |
| − | + | <ref group="Figure" name="cdaextensions"></ref> shows how the CDA model has been enhanced with the R_Medication CMET. | |
| − | + | {{IncludeImage|IPS extensions.png|700px|90%}} | |
| − | |||
| − | |||
| − | <ref group="Figure" name=" | + | <ref group="Figure" name="cdaextensions">CDA model has been enhanced with the R_Medication</ref>Extension of the CDA model from the content of the R_Medication CMET. |
| − | |||
| − | |||
| − | |||
| − | |||
== Provenance == | == Provenance == | ||
| Line 340: | Line 332: | ||
The approach proposed for this version of the IPS is to: | The approach proposed for this version of the IPS is to: | ||
| − | *Allow | + | *Allow optional documentation of section-level provenance. |
| − | *Require document-level provenance | + | *Require document-level provenance. |
*Define IPS document provenance as one of two types: human-curated or software-assembled, based on the authors recorded in the header. | *Define IPS document provenance as one of two types: human-curated or software-assembled, based on the authors recorded in the header. | ||
**The classification is based on whether the IPS document is constructed by a human or an automated process, regardless of whether the IPS contains some content of both kinds. | **The classification is based on whether the IPS document is constructed by a human or an automated process, regardless of whether the IPS contains some content of both kinds. | ||
*Require the IPS source system to identify the IPS provenance type and author. | *Require the IPS source system to identify the IPS provenance type and author. | ||
**The author shall be a human, if the IPS provenance type is "human-curated", or a device or system if the IPS provenance type is "software-assembled". | **The author shall be a human, if the IPS provenance type is "human-curated", or a device or system if the IPS provenance type is "software-assembled". | ||
| − | **In the case of a software-assembled IPS that is then verified by a human, the document provenance type shall be "software-assembled" and the author shall be the device or system that constructed the IPS document, but an additional verifier identity shall name the human who performed this check. For the avoidance of doubt, verifier is not the same as legalAuthenticator ( | + | **In the case of a software-assembled IPS that is then verified by a human, the document provenance type shall be "software-assembled" and the author shall be the device or system that constructed the IPS document, but an additional verifier identity shall name the human who performed this check. For the avoidance of doubt, verifier is not the same as legalAuthenticator (the verifier is represented as a participant with typeCode "VRF"). However, in cases where the verifying person intentionally wishes to sign the document, this shall be recorded as a legalAuthenticator. |
*Allow optional section level author, provenance type, verifier, and informant identification, for IPS source systems that can support this. | *Allow optional section level author, provenance type, verifier, and informant identification, for IPS source systems that can support this. | ||
*Not attempt to implement the US Realm CDA data provenance templates. | *Not attempt to implement the US Realm CDA data provenance templates. | ||
| − | Note: Discussions with the EHR work group suggest that a possible future project should be an IPS functional profile, once there is greater clarity and operational experience of using the IPS. | + | ''Note: Discussions with the EHR work group suggest that a possible future project should be an IPS functional profile, once there is greater clarity and operational experience of using the IPS.'' |
| − | |||
| − | |||
== Representation of Names == | == Representation of Names == | ||
| − | This specification requires that any Person Name is represented | + | This specification requires that any Person Name is represented including at least the given and family components and therefore is never documented as a single string. |
| − | Even | + | Even though it is recognized that there is not in all cultures the same concept of “family name”, no evidence has been collected in analyzing the international context (e.g. Japan, Korea; China) that justifies the retirement of this requirement. |
| − | Moreover, due to the global scope of the International Patient Summary, the case of non-alphabetic representations of the names | + | Moreover, due to the global scope of the International Patient Summary, the case of non-alphabetic representations of the names has also been considered. |
In this case, to facilitate the global use of the IPS, at least one alphabetic representation of the name SHALL be provided. | In this case, to facilitate the global use of the IPS, at least one alphabetic representation of the name SHALL be provided. | ||
Latest revision as of 09:53, 17 July 2018
Contents
- 1 Design conventions and principles
- 1.1 How to use terminologies (preferred binding)
- 1.2 Representing "known absent" and "not known"
- 1.3 Uncoded information
- 1.4 Unmapped Coded Concepts
- 1.5 Mapped coded concepts
- 1.6 Translation of designations
- 1.7 Narrative Translations
- 1.8 Determining the Status of Clinical Statement
- 1.9 The use of medication statements in the summary
- 1.10 Medicinal Product Identification
- 1.11 Provenance
- 1.12 Representation of Names
Design conventions and principles
How to use terminologies (preferred binding)
As stated in section 1.5, to be universally exchangeable the International Patient Summary must rely on international multilingual reference terminologies. To that effect, each codeable element of the international patient summary template is bound to a Value Set built upon an international reference terminology, such as SNOMED CT, LOINC, UCUM or EDQM Standard Terms. In some selected cases, in consideration of the availability of other globally usable reference terminologies and for alignment with a future FHIR version of the IPS, FHIR-defined terminologies have been specified. These terminologies have been selected to provide the preferred bindings for the codeable elements of the patient summary. They are the primary terminologies of this specification.
Nevertheless, it is anticipated that in some situations a system producing an instance of patient summary might not support one or the other of these primary terminologies, supporting only a local interface terminology instead. Similarly, it is also anticipated that the receiving system might in some cases not be able to use a code in a patient summary, either because this code belongs to a primary terminology that the receiving system does not support or because this code belongs to an interface terminology specific to the country of the producing system.
In order to maximize the international scope and usability of patient summaries, and also to accommodate the exceptional situations listed above, this specification makes these requirements:
- The Primary Code of a codeable element SHOULD be populated.
- If populated, the Primary Code of a codeable element SHALL be chosen from the primary terminology assigned to the value set bound to this element; unless exceptions have been agreed.
- The 'displayName' of the Primary Code SHALL be populated with a term representing this same code in the terminology used, in the language chosen for the current instance of the patient summary.
- When the primary 'code' element is not populated, an appropriate 'nullFlavor' value SHALL be used along with the 'originalText' element (referencing a textual expression in the narrative representing the meaning for the producer) and/or one or more coded 'translation' elements.
- One or more Alternate Codes from a local interface terminology MAY be provided, each with its associated 'displayName'.
- In case the primary code is derived from an alternate terminology the alternate code SHOULD be provided in the translation element.
Primary Code
In the data type for codeable elements (CD constrained by the CD.IPS template), the Primary Code is represented by the attributes @code, @displayName, @codeSystem, @codeSystemName, @codeSystemVersion.
Alternate Code
In the data type for codeable elements (CD constrained by the CD.IPS template), an Alternate Code is carried in a 'translation' sub-element.
Original Text
In the data type for codeable elements (CD constrained by the CD.IPS template), the Original Text is provided in the 'originalText' sub-element.
Representing "known absent" and "not known"
In line with the properties of minimalism and non-exhaustiveness for the IPS (see the IPS definition above), and benefiting from the experience acquired with the European cross-border services, this guide explicitly addresses two general situations:
- condition or activity unknown
- condition or activity known absent.
Other kinds of negations such as: (a) the negation of an allergy to a specific agent; (b) the absence of a particular disease; or (c) the fact that a specific vaccination has not been performed, have been considered beyond the set of essential data for an IPS.
This specification represents this core set of negations (“general condition/activity unknown” and “general condition/activity/known absent” ) using explicit coded elements rather than relying on specific mechanisms of HL7 CDA such as nullFlavor and negationInd attributes or human readable text (possibly not understood by the foreign country receiver).
In contrast to the practice to use negationInd or nullFlavor attributes on a section itself, we prohibit the use of these attributes on section level to express “unknown” or “no information” situations. A section holds the categorized (coded) narrative part of the documented activity and will never carry negationInd or nullFlavor attributes. Instead, we enforce by design, that “unknown” or “no information” expressions always go to the coded entry with a corresponding act code.
The main reasons for this choice are:
- @negationInd in CDA has been superseded in V3 later by two other negation indicators: @actNegationInd and @valueNegationInd.
- To make clinical content representation less dependent on a particular format or syntax, enabling a more practical path to transforming and exchanging data from one standard format (e.g. CDA R2) to another (e.g. FHIR).
- to provide one single method to express the presence or absence of a particular condition (e.g. an allergy) or activity (e.g. an immunization), or the lack of knowledge regarding this kind of condition or activity, resulting in a more robust and easily implementable specification.
For the other kinds of negations, not explicitly mentioned in this guide, it is suggested to apply – where possible – the same approach. Future versions of this guide may extend the number of cases covered and include new coded concepts for supporting them.
When needed, more specific statements such as the absence of a specific condition or activity, although considered as beyond the set of essential data for IPS, can still be expressed by using the native negationInd attribute of CDA R2.
Uncoded information
An IPS originator may not be able to value a coded element with an appropriate coded concept, but only with textual information. This may happen for two reasons:
- the originator is not able to express the concept in the reference value sets
- the originator is not able to express the concept in any known terminology.
The first case, assuming that the coding strength is Required (aka CNE, coded, no extensions), is represented in this guide with the following assertion:
<code codeSystem="2.16.840.1.113883.6.96" nullFlavor="OTH">
<originalText>
<reference value="#ref1"/>
</originalText>
</code>
That is expressing that there are no codes applicable in the referred code system (in the example, SNOMED CT). Please note that according to this guide the text is documented in the section narrative and only referenced by the coded element.
Note: Data Types R1 doesn't allow specifying that there are no codes applicable in the referred value set, as instead is possible with Data Types R2. Future versions of this guide may consider extending the data type to better support this situation.
The second case, that applies both to Required (aka CNE, coded no extensions) and Extensible (CWE, coded with extensions) coding strengths, is instead here represented valuing the coded element with the most generic nullFlavor “NI” (No Information) and pointing the text in the section narrative:
<value xsi:type="CD" nullFlavor="NI">
<originalText>
<reference value="#ref1"/>
</originalText>
</value>
Note: The most proper NullFlavor code to be used here would be "UNC" (Uncoded). This code is available in the current and other recent versions of the HL7 RIM, but it is not present in version 2.07 of the RIM on which the CDA R2 standard is based. In the absence of "UNC", the most appropriate NullFlavor code to use for representing that the data is unable to be coded is "NI".
Unmapped Coded Concepts
In several real world situations the records used as source for the Patient Summaries may use locally adopted terminologies mapped into the reference value sets. When the original coded concept cannot be mapped in one of the coded concepts included in the reference value sets, it is recommended to populate the original code in the IPS instance (in a 'translation' sub-element), with a nullFlavor indicating that the mapping didn’t occur. (See also the Concept code mapping in the functional requirements section.). The nullFlavor value depends upon the coding strength of the binding.
Two circumstances are considered here: the case in which the coding strength is Required (CNE) and when it is Extensible (CWE).
In the case of coding strength Required (CNE), use nullFlavor "OTH":
<value xsi:type="CD" codeSystem="2.16.840.1.113883.6.96" nullFlavor="OTH">
[
<originalText>
<reference value="#ref1"/>
</originalText>
]
<translation code="A02.9" codeSystem="2.16.840.1.113883.6.3"
displayName="Infezioni da Salmonella non specificate"/>
</value>
The square brackets [ ] are used to indicate that the originalText element may or may not be present
Note: It may happen that a mapping would be possible in the target code system, but not in the target value set; Data Types R1 does not allow the specification of this difference, that there are no codes applicable in the reference value set, which is possible with Data Types R2.
Future versions of this guide may consider extending the data type to better support this situation.
In the case of Extensible (CWE) coding strength, this guide recommends representing the original code in the <translation> sub-element and using a nullFlavor for the primary code. This highlights the fact that a mapping to the reference value set was attempted, but no suitable target codes were identified.
<value xsi:type="CD" codeSystem="2.16.840.1.113883.6.96" nullFlavor="NI">
[
<originalText>
<reference value="#ref1"/>
</originalText>
]
<translation code="A02.9" codeSystem="2.16.840.1.113883.6.3"
displayName="Infezioni da Salmonella non specificate"/>
</value>
The square brackets [ ] are used to indicate that the originalText element may or may not be present.
Mapped coded concepts
As mentioned above, in several circumstances an original coded concept is mapped into the reference value set. When this occurs, both the original and the reference codes should be reported in the IPS instance.
Functional requirements exposed in section 3.1 (multi-coding, link to original text, mapping between codes of the same code system) are also detailed below.
Case 1: Single local code mapped to primary code from the reference value set.
<value xsi:type="CD" code="42338000" codeSystem="2.16.840.1.113883.6.96"
displayName="Salmonella gastroenteritis">
[
<originalText>
<reference value="#ref1"/>
</originalText>
]
<translation code="003.0" codeSystem="2.16.840.1.113883.6.103"
displayName="Gastroenterite da Salmonella"/>
</value>
The square brackets [ ] are used to indicate that the originalText element may or may not be present
Case 2: Multiple local codes mapped together using nested 'translation' elements, and mapped to the primary code from the reference value set.
<value xsi:type="CD" code="422479008" codeSystem="2.16.840.1.113883.6.96"
codeSystemName="SNOMED CT"
displayName="FEMALE BREAST INFILTRATING DUCTAL CARCINOMA, STAGE 2">
[
<originalText>
<reference value="#problem4name"/>
</originalText>
]
<translation code="code-example" codeSystem="1.999.999"
codeSystemName="this is only an example"
displayName="FEMALE BREAST INFILTRATING DUCTAL CARCINOMA, STAGE 2">
<translation code="174.9" codeSystem="2.16.840.1.113883.6.103"
codeSystemName="ICD-9CM"
displayName="Malignant neoplasm of breast (female), unspecified"/>
<translation code="C50.919" codeSystem="2.16.840.1.113883.6.90"
codeSystemName="ICD-10-CM"
displayName="Malignant neoplasm of unspecified site of unspecified female breast"/>
</translation>
</value>
Case 3: Original and the reference coded concepts belong to the same code system (distinct codes). This may be the result of a different level of granularity between the original term and the reference value sets.
<code code="60591-5" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" displayName="Patient Summary">
<translation code="60592-3" codeSystem="2.16.840.1.113883.6.1"
displayName="Patient summary unexpected contact"/>
</code>
Note: The R1 data type definition identifies the <translation> as “a set of other concept descriptors that translate this concept descriptor into other code systems." There is however a common understanding that it may be more than one representation in a single code system where code systems allow multiple representations, such as SNOMED CT. Data Types R2 extended the possibility to provide translations also in the same code system.
Translation of designations
The capability of recording one or more designations, in different languages, for the exchanged Patent Summary is one of the functional requirements requested for “safety and liability reasons” by the European Cross-border services (see Designations’ Translation under the Functional requirements and high-level use cases for more details).
Given that the base CDA R2 standard which uses datatypes R1 does not offer a native way to convey the language translation of 'displayName', this guide introduces an optional 'ips:designation' extension to the CD datatype for that purpose.
Below are examples of usage of this extension.
No code mapping
<code code="60591-5" codeSystem="2.16.840.1.113883.6.1"
codeSystemName="LOINC" displayName="Patient Summary">
<ips:designation language="it-IT">Profilo Sanitario Sintetico</ips:designation>
<ips:designation language="fr-FR">Patient Summary</ips:designation>
<ips:designation language="en">Patient Summary</ips:designation>
</code>
Including code mapping
<value xsi:type="CD" code="42338000" codeSystem="2.16.840.1.113883.6.96"
displayName="Salmonella-gastroenterit">
<ips:designation language="da-DK">Salmonella-gastroenterit</ips:designation>
<ips:designation language="en">Salmonella gastroenteritis (disorder)</ips:designation>
[
<originalText>
<reference value="#ref1"/>
</originalText>
]
<translation code="003.0" codeSystem="2.16.840.1.113883.6.103"
displayName="Gastroenterite da Salmonella"/>
</value>
Narrative Translations
“Narrative translation” means both the translation of the original narrative text, that can be human curated or automatically performed, and the generation of a translated narrative based on the coded entries.
The functional requirements associated with this process are described in the Designations’ Translation section under Functional requirements and high-level use cases, and can be summarized in two main points : (a) language identification and (b) distinguishable original and translated narratives. Moreover, the methodology applied for the narrative translation (e.g. derived from the coded entries; translated by a generic service;..) should be noted.
Regarding the translation of section narrative <text>, this guide recommends providing this translation on purely textual subordinate sections (one per translation) as specified in the IPS Translation Section (2.16.840.1.113883.10.22.3.15) template.
An example of this is:
<section>
<templateId root="2.16.840.1.113883.3.1937.777.13.10.5"/>
<id root="..." extension="..."/>
<code code="48765-2" codeSystem="2.16.840.1.113883.6.1"
displayName="Allergies and adverse reactions"/>
<title>Allergies and Intolerances</title>
<text>No known Allergies</text>
<!-- omissions -->
<component>
<section>
<!-- subordinate section carrying a translation of the parent section -->
<title>Allergie ed Intolleranze</title>
<text>Nessuna Allergia Nota</text>
<languageCode code="it-IT"/>
</section>
</component>
</section>
Determining the Status of Clinical Statement
Note: Most of the description provided in this section is copied from the § 3.3 Determining the Status of Clinical Statement of the C-CDA DSTU R2.1 Implementation Guide Volume 1.[1]
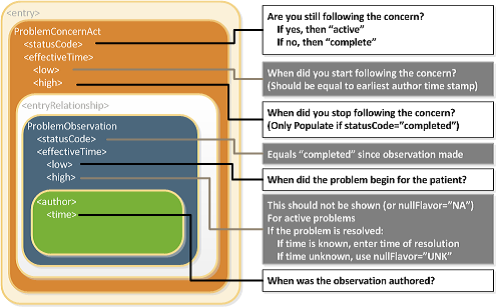
A recipient must be able to determine whether the status of an entry — which can include a problem, a medication administration, etc. — is active, completed, or in some other state. Determination of the exact status is dependent on the interplay between an act’s various components (such as statusCode and effectiveTime). The following principles apply when representing or interpreting the status of a clinical statement.
- The Act.statusCode of the clinical statement specifies the state of the entry: Per the RIM, the statusCode “reflects the state of the activity. In the case of an Observation, this is the status of the activity of observing, not the status of what is being observed.”
- Act.statusCode and Act.moodCode are inter-related: Generally, an act in EVN (event) mood is a discrete event (a user looks, listens, measures, and records what was done or observed), so generally an act in EVN mood will have a statusCode of “completed.” A prolonged period of observation is an exception, in which a user would potentially have an observation in EVN mood that is “active.” For an Observation in RQO (request) mood, the statusCode generally remains “active” until the request is complete, at which time the statusCode changes to “completed.” For an Observation in GOL (goal) mood, the statusCode generally remains “active” as long as the observation in question is still an active goal for the patient.
- Act.statusCode and Act.effectiveTime are interrelated: Per the RIM, the effectiveTime, also referred to as the “biologically relevant time,” is the time at which the act holds for the patient. So, whereas the effectiveTime is the biologically relevant time, the statusCode is the state of the activity. For a provider seeing a patient in a clinic and observing a history of heart attack that occurred 5 years ago, the status of the observation is completed, and the effectiveTime is five years ago.
The IPS Problem Concern Entry and the IPS Allergy and Intolerance Concern templates reflect an ongoing concern on behalf of the provider that placed the concern (e.g. a disease, an allergy, or other conditions) on a patient’s problem or allergy list. The purpose of the concern act is that of supporting the tracking of a problem or a condition (e.g. an allergy). A concern act can contain one or more discrete observations related to this concern. Each of them reflects the change in the clinical understanding of a condition over time. For instance, a Concern may initially contain a symptom of “chest pain”, later identified as consequence of a gastroesophageal reflux. The later problem observation will have a more recent author time stamp.
There are different kinds of status that could be of clinical interest for a condition:
- The status of the concern (active, inactive,..)
- The status of the condition (e.g. active, inactive, resolved,..)
- The confirmation status [verification status, certainty] (e.g. confirmed, provisional, refuted,…)
Not all of them can be represented in a CDA using the statusCode elements of the concern (ACT) and observation (condition).
Status of the concern and related times
The statusCode of the Problem Concern Act is the definitive indication of the status of the concern. So long as the provider has an ongoing concern — meaning that the provider is monitoring the condition, whether it includes problems that have been resolved or not — the statusCode of the Concern Act is “active.” When the underlying conditions are no longer an active concern, the statusCode of the Problem Concern Act is set to “completed.”
The Concern Act effectiveTime reflects the time that the underlying condition was considered a concern. It may or may not correspond to the effectiveTime of the condition (e.g., even five years later, the clinician may remain concerned about a prior heart attack). The effectiveTime/low of the Concern Act asserts when the concern became active. This equates to the time the concern was authored in the patient's chart. (i.e. it should be equal to the earliest author time stamp) The effectiveTime/high asserts when the concern became inactive, and it is present if the statusCode of the concern act is "completed". If this date is not known, then effectiveTime/high will be present with a nullFlavor of "UNK".
Status of the condition and related times
Each Observation contained by a Concern Act is a discrete observation of a condition. Its statusCode is always “completed” since it is the “status of the activity of observing, not the status of what is being observed”. The clinical status of a condition (e.g. a disease, an allergy,..) is instead recorded by specialized subordinated observations (IPS Allergy Status Observation; IPS Problem Status Observation), documenting whether it is active, in remission, resolved, et cetera. The effectiveTime, also referred to as the "biologically relevant time", is the time at which the observation holds for the patient. For a provider seeing a patient in the clinic today, observing a history of penicillin allergy that developed six months ago, the effectiveTime is six months ago. The effectiveTime of the Observation gives an indication of whether or not the underlying condition is resolved, but the current status is documented by a subordinated observation. The effectiveTime/low (a.k.a. "onset date") asserts when the allergy/intolerance became biologically active. The effectiveTime/high (a.k.a. "resolution date") asserts when the allergy/intolerance was biologically resolved. If the date of resolution is not known, then effectiveTime/high will be present with a nullFlavor of "UNK".
[Figure 1] Problem Concern Act (from C-CDA IG DTSU R2.1)[1]
Confirmation status
The confirmation status, also noted as verification status or certainty, indicates the certainty associated with a condition (e.g. confirmed, provisional, refuted,…) providing information about the potential risk, for example, of a reaction to the identified substance. The confirmation status of a condition (e.g. a disease, an allergy,..) is recorded by a specialized subordinated observation (IPS certainty Observation), documenting whether the condition is confirmed, unconfirmed, provisional, refuted, et cetera.
The use of medication statements in the summary
A medication list may strongly vary depending on the context of use (e.g. support for prescription or dispensation, drug reconciliation, etc. ) and on the type of information reported (e.g. patient-reported medication, prescribed, dispensed or administered medications, active or past medications, etc.).
This is still true also for the medication summary in a Patient Summary. It could be, for instance, a list of "Relevant prescribed medicines whose period of time indicated for the treatment has not yet expired whether it has been dispensed or not" (European guidelines on Patient Summary [2]); a list of actually dispensed medications; a list of relevant medications for the patient (IHE PCC [3]); or conversely, it could be a complete history including the full patient's prescription and dispensation history and information about intended drug monitoring (C-CDA [1]).
Moreover, for the scope of the International Patient Summary, it is important to know what medications are being taken by or have been given to a patient; without necessarily providing all the details about the medication order, supply, administration or monitoring. This information need, in line with the IPS principle of minimum non exhaustive data, is well expressed by the concept of Medication Statement (see https://www.hl7.org/fhir/medicationstatement.html).
The IPS medication summary is therefore a list of relevant medication statements, possibly built from either a prescription list or a dispensation list. In fact, in many practical cases data included in a Patient Summary are derived from the list of the medicines prescribed by a GP and recorded in its EHR-S; or extracted from a in regional/national prescribing and/or dispensation systems. In these cases, data obtained from actual dispensation and/or prescription records can be still recorded in the IPS as statements and the original request, supply or administration records referred by the statement if really needed.
Medicinal Product Identification
The identification of medicinal products is quite easily solved within a single jurisdiction relying on local drug databases. In contrast, it is one of the major open issues for eHealth services across jurisdictions.
The set of ISO standards called IDMP[4] - designed initially for the regulatory scope, but hopefully extensible to other domains - are the most promising solution for solving this known issue, as also highlighted by the European project OpenMedicine [5]. The completion of the IDMP implementation guides, the deployment of the needed supporting services, and the development of some companion standards that will allow the seamless flow of the IDMP identifiers and attributes from the regulatory space to the clinical world (and back) are however still in progress. Additional time is needed before these identifiers and attributes will be available in full for practical use.
Following therefore the IPS principles of “implementability”, “openness" and "extensibility”, the solution proposed here does not rely on IDMP identifiers. Nonetheless, it takes into account, however, relevant IDMP identifiers and attributes which are already usable in the IPS, namely the Pharmaceutical Product Identifiers (PhPIDs), the Medicinal Product Identifier (MPID), and the Medicinal Product Package Identifier (PCID).
Note: IDMP Medicinal Product (MPID) and Medicinal Product Package (PCID) identifiers depend on the market authorization. The “same” product might therefore have different IDs if different authorizations have been received in different countries, while the PhPID should be the same. For the purpose of the IPS, future standards and implementation guides should define global product identifiers that do not depend on the drug registration process (as the Virtual Medicinal Product in SNOMED CT) and relate to IDMP identifiers.
Thus, in the absence of a global identification system for medicinal products, the solution proposed here is based on the approach initially adopted by the European cross-border services (epSOS and currently by the eHDSI project), reused by the IHE Pharmacy templates and more recently adopted (for specific cases) by the HL7 Pharmacy Medication statement templates. The main idea is to integrate local drug identifiers (e.g. product codes) with all the relevant identifying and descriptive attributes that may help the receiver to understand the type of product the sender is referring to, e.g.: active ingredients, administrative dose forms, strength, route of administration and package description.
Medication data is usually represented in the CDA Templates using the manufacturedMaterial class, which includes a code and a name to describe any level of the product: packaged product, medicinal product, classes or clusters or products, and so on. This information is not however sufficient for covering the requirements of the IPS.
[Figure 2] Representation of medicines in CDA
Hence, in order to describe these attributes the CDA model has been extended enhancing the Manufactured Material class with attributes and relationships derived from the latest published version of the R_Medication CMET (see HL7 V3 Normative Edition 2017) based on the HL7 Common Product Model (“The common product model is used to improve the alignment between the different representations of products used within the body of HL7 Version 3 models. One goal of this effort is to make it possible to have a single representation, which could then be used as a common message element type (CMET) across those models.”) .
[Figure 3] shows how the CDA model has been enhanced with the R_Medication CMET.
[Figure 3]Extension of the CDA model from the content of the R_Medication CMET.
Provenance
In the development of this Implementation Guide, consideration was given to the HL7 CDA® Release 2 Implementation Guide: Data Provenance, Release 1 - US Realm Draft Standard for Trial Use (December 2015). That guide provides a matrix offering a thorough and systematic analysis of provenance characteristics of electronic health records. Given the agreed scope principle that the IPS be minimal and implementable, and the variable maturity and operational methods of existing national patient summaries, the proposal is that this first version should not attempt to require the full detail of that provenance specification.
The approach proposed for this version of the IPS is to:
- Allow optional documentation of section-level provenance.
- Require document-level provenance.
- Define IPS document provenance as one of two types: human-curated or software-assembled, based on the authors recorded in the header.
- The classification is based on whether the IPS document is constructed by a human or an automated process, regardless of whether the IPS contains some content of both kinds.
- Require the IPS source system to identify the IPS provenance type and author.
- The author shall be a human, if the IPS provenance type is "human-curated", or a device or system if the IPS provenance type is "software-assembled".
- In the case of a software-assembled IPS that is then verified by a human, the document provenance type shall be "software-assembled" and the author shall be the device or system that constructed the IPS document, but an additional verifier identity shall name the human who performed this check. For the avoidance of doubt, verifier is not the same as legalAuthenticator (the verifier is represented as a participant with typeCode "VRF"). However, in cases where the verifying person intentionally wishes to sign the document, this shall be recorded as a legalAuthenticator.
- Allow optional section level author, provenance type, verifier, and informant identification, for IPS source systems that can support this.
- Not attempt to implement the US Realm CDA data provenance templates.
Note: Discussions with the EHR work group suggest that a possible future project should be an IPS functional profile, once there is greater clarity and operational experience of using the IPS.
Representation of Names
This specification requires that any Person Name is represented including at least the given and family components and therefore is never documented as a single string.
Even though it is recognized that there is not in all cultures the same concept of “family name”, no evidence has been collected in analyzing the international context (e.g. Japan, Korea; China) that justifies the retirement of this requirement.
Moreover, due to the global scope of the International Patient Summary, the case of non-alphabetic representations of the names has also been considered.
In this case, to facilitate the global use of the IPS, at least one alphabetic representation of the name SHALL be provided.
- ↑ 1.0 1.1 1.2 HL7 C-CDA Implementation Guide DSTU R2.1 http://www.hl7.org/implement/standards/product_brief.cfm?product_id=379
- ↑ EHN Guideline on the electronic exchange of health data under Cross-Border Directive 2011/24/EU. Release 2. (https://ec.europa.eu/cefdigital/wiki/display/EHOPERATIONS/Business+Analysis+and+Requirements+Management?preview=/55878732/55878698/(Adopted)%20Patient%20Summary%20Guideline%20cross-border%20exchange%20of%20health%20data%20(release%202).pdf
- ↑ IHE Patient Care Coordination Technical Framework http://ihe.net/Technical_Frameworks/#pcc
- ↑ IDMP standards https://www.idmp1.com/idmp-standards
- ↑ European project OpenMedicine http://www.open-medicine.eu/home.html
Cite error: <ref> tags exist for a group named "Figure", but no corresponding <references group="Figure"/> tag was found, or a closing </ref> is missing