IPS Functional requirements 1
Contents
Functional requirements and high-level use cases
Several cases may be identified for the International Patient Summary within its scope (“specialty-agnostic, condition-independent, but readily usable by clinicians for the cross-border unscheduled care”). Section Examples (in progress) provides examples of some real world user stories for the cross-border care developed by the Trillium Bridge Project and by the S&I EU-US eHealth Cooperation Initiative in the scope of the EU/US Roadmap initiative.
The cross-border care is not however the only expected usage scenario for the IPS: some European countries have already manifested their interest on the IPS work for their future national Patient Summary for unscheduled care.
As shown by the user stories there are several possible options for the IPS in term of creation, sharing and usage of an IPS, for example:
- an IPS could be a created when requested and used (before or during a care episode); or can be asynchronously generated and made available for future usage (e.g. store and retrieve).
- the IPS can be retrieved using a document exchange infrastructure; transported by the patient; or shared using cloud-services.
- the IPS may be subject of a transformation process, that may include syntactical conversions; coded concepts mappings and coded concept designation or free text translations. This transformation process may be performed in the creation phase; during the transmission or after the reception for example using an external service.
- Finally, the received CDA may be expected to be displayed using a common CDA stylesheet; may be used to extract some relevant information to be displayed by the EHR-S or to be incorporated into the receiver’s EHR; or a specialized viewer may be adopted to enable the display of the translated content.
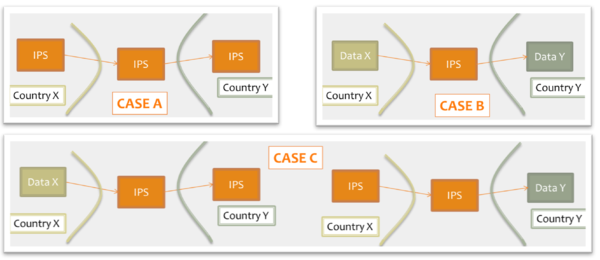
Moreover for cross-organizations/jurisdictions exchange the IPS could be used as:
- shared format among jurisdictions (case A): jurisdictions originate and use IPS conformant documents;
- pivot document among existing summaries / data formats (case B): for example as intermediate format between the US C-CDA CCD (Please note that the CCD scope differs from that of the IPS) and the European eHDSI Patient Summary for a Transatlantic Patient Summary exchange.
- Or in a mixed mode (Case C): either the originator or the consumer is expected to use an IPS conformant document.
[Figure 1]Examples of IPS usage
Considering all those possible combinations and additional business requirements agreed by jurisdictions, there are several technical infrastructures and services that may be designed to support these requirements.
It is out of scope of this standard to provide any indication about solutions and strategies for the IPS creation, sharing, syntactical and semantic mapping, translation, use.
Having said that an International Patient Summary may be characterized by:
- being the result of an automatic assembling (assembled IPS) or of a human summarization act (human curated IPS)
- having one or more EHR sources
- document information from a single or multiple jurisdictions/organizations
- being the result of a single or multiple encounters
A clear determination of such a kind of contextual information helps the comprehension and the trustiness on the received IPS. Most of these aspects are related to the data provenance, introduced in section Provenance above.
Even if in many jurisdictions require that only one active Patient Summary for unscheduled care would be made available; no constraints on this purpose are imposed by this guide being this strongly dependent on the case for which this template is used.
Moreover it is also out of scope for this guide to:
- provide guidance on how to determine the relevancy of data for their inclusion in a IPS;
- define selection or composition rules for facing potential inconsistencies from multiple sources in case of automatic collection.
Code mappings and multilinguistic support
The capability of managing locally used coded concepts and reference terminologies, and that of providing receiving providers with human readable information in a language that can be understood by them, are critical aspects to be taken in account for the cross-border sharing of documents. This section summarizes some of the requirements related to these aspects, including also additional needs derived from the European cross-border services and some lessons learned by the EU/US Trillium Bridge Project. The European cross-border services (eHDSI see link to eHDSI) use a business to business exchange infrastructure based on a network of countries’ gateways that hide the national infrastructures; the eHDSI Patient Summary (eHDSI PS) is used as a “pivot” document for the cross-country exchange: local data/document formats are in fact remapped into the eHDSI PS; the document exchanged is processed each time it passes through one of these a gateway applying the needed syntactical transformations; codes mapping; codes designations’ translation; finally, in the current practice the received PSs are displayed using specialized display tools, that build a human readable representation of the PS in the target language using the translated designations reported in the coded entries. The adoption of translated narratives in the received document has been one of the indications received by the Trillium Bridge Project. This in fact allows extending the multi-linguistic support for the cross-border patient summaries to a wider set of potential consumers (EHR-Systems), without requiring specialized viewers as applied in the European services.
Concept code mapping
In several real world cases the records used as source for the Patient Summaries may use locally adopted terminologies that – when possible – are remapped into the reference value sets; or may be able in some cases to provide only uncoded information. This leads to a series of requirements for the IPS that will be discussed in the Design conventions and principles section.
- When the original coded concept is mapped in one of the coded concepts included in the reference value sets (called hereafter reference code/coded concept), both the original and the reference codes should be reported in the IPS instance.
- When the original coded concept is not mapped in one of the coded concepts included in the reference value sets, the original code should be reported in the IPS instance as well as the indication that the mapping didn’t occur.
- When the original record, for a specific coded element, is not able to provide coded but only textual information, this information should be preserved in the IPS instance.
Moreover it should be taken in account that:
- The original record may support multiple coding, so that the IPS instance should be able to distinguish if the additional codes belong to the original content or they are the result of a coded concepts mapping.
- The original record may include references to the pieces of text the coding was derived from, if present, the IPS instance should preserve this link between the original code and the referred text.
- Distinct original and the reference coded concepts may belong to the same code system. This may be the result of a different level of granularity between the original and the reference value sets; or of a format transformation (e.g. a CCD document is used as input for generating an IPS). Also for these cases the requirement of recording both coded concepts should be applied.
Note: in some jurisdictions (as the European Cross-border services) some of these "should" may be turned into a "shall".
Multilinguistic support
The debate on the multi linguistic support for the IPS could be split in two distinct subjects:
- The translation of coded concept designations (displayName)
- The translation of the narrative
In both cases several options may be identified depending also on the use cases considered:
- The translation in the receiving country language: an IPS is prepared before a planned travel; a foreign provider retrieves a translated copy of the IPS from the patient country of affiliation…
- The translation into a commonly agreed language: an English version of the IPS is prepared...
- The predefined set of translations is included in the shared IPS...
It is out of the scope of this guide to suggest any of these solutions: all of them should be supported by this standard.
Designations’ Translation
Basically, the requirements related to the translation of the designations derive from the European Cross-border services imposing as functional requirements that for “safety and liability reasons” that all the terms (designations, displayName) associated to the original codes in the original language shall be recorded in the exchanged documents; together with the English and the receiving country language terms (designations, displayName) associated to the reference codes. The designations translated in the receiving country language are used for generating the human readable content shown to the receiving provider. No free text translation is applied in this case. In order to accomplish this need, the IPS should have the capability of recording one or more designations, possibly indicating the language used.
Narrative Translation
With the term narrative translation two different concepts are covered:
- The translation of the original narrative text. This can be in principle:
- Human curated
- Automatically performed (e.g. using specialized or generic translation services)
- The creation of a new translated narrative based on the coded entries.
Different levels of quality and liability can be obtained depending on the solution adopted and on the translation service used. It is out of the scope of this guide to suggest any of these solutions, in all the cases however:
- the language of the narrative should be identifiable
- the original and the translated narrative should be clearly distinguished
- the methodology applied for the narrative translation (e.g. derived from the coded entries; translated by a generic service;..) should be recognizable
Cite error: <ref> tags exist for a group named "Figure", but no corresponding <references group="Figure"/> tag was found, or a closing </ref> is missing