Difference between revisions of "IPS Introduction 1"
(→General Principles for this Specification) |
(→Introduction) |
||
| (59 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
=Introduction= | =Introduction= | ||
| − | + | An International Patient Summary (IPS) document is an electronic health record extract containing essential healthcare information intended for use in the unscheduled, cross-border care scenario, comprising at least the required elements of the IPS dataset. The IPS dataset is '''''a minimal and non-exhaustive patient summary dataset, specialty-agnostic, condition-independent, but readily usable by clinicians for the cross-border unscheduled care of a patient.''''' | |
==Purpose== | ==Purpose== | ||
| Line 7: | Line 7: | ||
The goal of this Implementation Guide is to identify the required clinical data, vocabulary and value sets for an international patient summary. | The goal of this Implementation Guide is to identify the required clinical data, vocabulary and value sets for an international patient summary. | ||
The international patient summary is specified as a templated document using HL7 CDA R2. | The international patient summary is specified as a templated document using HL7 CDA R2. | ||
| − | The primary use case is to provide support for cross-border or | + | The primary use case is to provide support for cross-border or cross-juridictional emergency and unplanned care. |
This specification aims to support: | This specification aims to support: | ||
| Line 17: | Line 17: | ||
== Project Background == | == Project Background == | ||
| − | This Implementation Guide has drawn upon the results of multiple previous projects on patient summaries (including but not limited to epSOS, ONC S&I, Trillium Bridge, Sequoia eHealth Exchange), rules and recommendations for vocabularies and value sets (in multilingual settings) and templates for the implementation of international patient summary documents. | + | This Implementation Guide has drawn upon the results of multiple previous projects on patient summaries (including but not limited to epSOS <ref>The epSOS Project http://epsos.eu/</ref>, ONC S&I, Trillium Bridge<ref>The Trillium Bridge Project http://www.trilliumbridge.eu</ref>, Sequoia eHealth Exchange <ref>The Sequoia Project https://sequoiaproject.org/</ref>), rules and recommendations for vocabularies and value sets (in multilingual settings) and templates for the implementation of international patient summary documents. |
| − | The idea of the International Patient Summary has been one of the main results of the 2010 EU/US Memorandum of Understanding through its two operational arms: the European project Trillium Bridge | + | The idea of the International Patient Summary has been one of the main results of the 2010 EU/US Memorandum of Understanding through its two operational arms: the European project Trillium Bridge and the Interoperability of EHR work group formed under the ONC Standards and Interoperability Framework (ONC S&I) EU/US eHealth Cooperation Initiative<ref>Memorandum of Understanding between the United States Department of Health and Human Services and the European Commission on Cooperation Surrounding Health Related Information and Communication Technologies http://ec.europa.eu/newsroom/dae/document.cfm?doc_id=1784</ref>. |
These initiatives identified the need for common templates and vocabularies for the patient summary. | These initiatives identified the need for common templates and vocabularies for the patient summary. | ||
| − | The Joint Initiative Council (JIC) on SDO Global Health Informatics Standardization has initiated the standard sets project with patient summary as its pilot <ref>http://www.jointinitiativecouncil.org/news/JIC_Standards_Set_development_20160101_v1.00.pdf</ref>; and the IPS became one of the main subjects of the new EU / US roadmap , having as declared goal “to enable a standardized international patient summary (IPS) to be in use by 2020”<ref>Transatlantic eHealth/health IT Cooperation Roadmap http://ec.europa.eu/newsroom/dae/document.cfm?doc_id=12123</ref>. | + | The Joint Initiative Council (JIC) on SDO Global Health Informatics Standardization has initiated the standard sets project with patient summary as its pilot <ref>http://www.jointinitiativecouncil.org/news/JIC_Standards_Set_development_20160101_v1.00.pdf</ref>; and the IPS became one of the main subjects of the new EU / US roadmap , having as a declared goal “to enable a standardized international patient summary (IPS) to be in use by 2020”<ref>Transatlantic eHealth/health IT Cooperation Roadmap http://ec.europa.eu/newsroom/dae/document.cfm?doc_id=12123</ref>. |
| − | The first standardization activity concerning the IPS was initially promoted in April 2014 by ONC within HL7 International. The project was called “INTernational PAtient Summary (INTERPAS)”. In May 2016, the European Commission | + | The first standardization activity concerning the IPS was initially promoted in April 2014 by ONC within HL7 International. The project was called “INTernational PAtient Summary (INTERPAS)”. In May 2016, the European Commission granted an Agreement with CEN/ TC 251, recognizing the need to effectively support the leadership and active participation in IPS standardization activities. Thanks to the new boost from the European Commission (EC) and ONC a revision of the HL7 project was started in May 2016, as well as the standardization activities in CEN/TC 251 for the European standards on Patient Summaries. |
Since the beginning of this new phase, the initiatives were envisaged as a '''single common IPS project''' supported by different organizations; where the CEN/TC 251 and the HL7 teams worked together, taking in account the inputs of the JIC Standard Sets initiative on Patient Summary, with the common intent of developing coherent set of standards to support the International Patient Summary concept. | Since the beginning of this new phase, the initiatives were envisaged as a '''single common IPS project''' supported by different organizations; where the CEN/TC 251 and the HL7 teams worked together, taking in account the inputs of the JIC Standard Sets initiative on Patient Summary, with the common intent of developing coherent set of standards to support the International Patient Summary concept. | ||
| − | To expedite progress it was also agreed to set up an informal collaboration, promoting a continuous alignment process between the two SDO-specific projects, thanks also to a cross-participation in the project teams. Overlaps have thus been minimized: the CEN/TC 251 activities have been focused on the IPS dataset, formalized by the CEN/TC 251 European standard ( | + | To expedite progress it was also agreed to set up an informal collaboration, promoting a continuous alignment process between the two SDO-specific projects, thanks also to a cross-participation in the project teams. Overlaps have thus been minimized: the CEN/TC 251 activities have been focused on the IPS dataset, formalized by the CEN/TC 251 Draft European standard (prEN) 17269:2018: ''The Patient Summary for Unscheduled, Cross-border Care"'' (the CEN/TC 251 prEN 17269:2018 PS in <ref group="Figure" name="saif"></ref>); the HL7 ones initially on its implementation based on HL7 CDA R2 - this guide - and next on FHIR (the HL7 IPS IGs in <ref group="Figure" name="saif"></ref>). The figure shows how the products of these standardization activities are placed in the HL7 SAIF Interoperability Matrix. |
{{IncludeImage|IPS_SAIF.png|600px|90%}} | {{IncludeImage|IPS_SAIF.png|600px|90%}} | ||
| − | <ref group="Figure" name="saif">Standards in the HL7 SAIF Interoperability Matrix</ref> Standards in the HL7 SAIF Interoperability Matrix | + | <ref group="Figure" name="saif">IPS Standards in the HL7 SAIF Interoperability Matrix</ref> IPS Standards in the HL7 SAIF Interoperability Matrix |
A formal agreement between HL7 International and CEN/TC 251 has been finally signed in April 2017 in which these organizations established “''in order to further the care for citizens across the globe <…> to collaborate on a single, common International Patient Summary (IPS) specification''”; and that “''the IPS specification shall focus on a minimal and non-exhaustive Patient Summary dataset, which is specialty-agnostic and condition-independent, but still clinically relevant.''”. | A formal agreement between HL7 International and CEN/TC 251 has been finally signed in April 2017 in which these organizations established “''in order to further the care for citizens across the globe <…> to collaborate on a single, common International Patient Summary (IPS) specification''”; and that “''the IPS specification shall focus on a minimal and non-exhaustive Patient Summary dataset, which is specialty-agnostic and condition-independent, but still clinically relevant.''”. | ||
| Line 41: | Line 41: | ||
*but readily usable by clinicians for cross-border unscheduled care of a patient. | *but readily usable by clinicians for cross-border unscheduled care of a patient. | ||
| − | In this context, ''minimal and non-exhaustive'' means that an IPS is not intended to reproduce (the entire) content of an Electronic Health Record (EHR) | + | In this context, ''minimal and non-exhaustive'' means that an IPS is not intended to reproduce (the entire) content of an Electronic Health Record (EHR). |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
''Specialty-agnostic'' means that an IPS is not filtered for a specialty. As an example, allergies are not filtered to the specialty of internal medicine, thus may also include food allergies, if considered to be relevant for, e.g. unplanned care. | ''Specialty-agnostic'' means that an IPS is not filtered for a specialty. As an example, allergies are not filtered to the specialty of internal medicine, thus may also include food allergies, if considered to be relevant for, e.g. unplanned care. | ||
| − | ''Condition-independent'' means that an IPS | + | ''Condition-independent'' means that an IPS is not specific to particular conditions, and focuses on the patient current condition(s). |
| − | Furthermore the scope of the IPS is global. Although this is a major challenge, this implementation guide takes various experiences and newer developments into account to address global feasibility | + | Furthermore the scope of the IPS is global. Although this is a major challenge, this implementation guide takes various experiences and newer developments into account to address global feasibility as far as possible. |
== General Principles for this Specification== | == General Principles for this Specification== | ||
| Line 61: | Line 54: | ||
{{IncludeImage|IPS_principles.png|300px|30%}} | {{IncludeImage|IPS_principles.png|300px|30%}} | ||
| − | <ref group="Figure" name="ipsprinciples"> The IPS Principles</ref>The IPS Principles | + | <ref group="Figure" name="ipsprinciples"> The IPS Principles</ref> The IPS Principles |
#The standards specification for the IPS will be implementable | #The standards specification for the IPS will be implementable | ||
| Line 75: | Line 68: | ||
#*The IPS provides common content that can be extended and specialized for other use cases, or localized for specific jurisdictional needs | #*The IPS provides common content that can be extended and specialized for other use cases, or localized for specific jurisdictional needs | ||
#*The IPS is open to emerging solutions for unresolved issues or improvements | #*The IPS is open to emerging solutions for unresolved issues or improvements | ||
| − | #The standards | + | #The standards specification and their implementation must be sustainable through: |
#*A robust maintenance and update process for the IPS | #*A robust maintenance and update process for the IPS | ||
#*A process to ensure clinical validity of the IPS, meeting: | #*A process to ensure clinical validity of the IPS, meeting: | ||
| Line 82: | Line 75: | ||
#**information quality requirements | #**information quality requirements | ||
| − | Moreover HL7 International and CEN/TC 251 will manage the expectations of the IPS standards | + | Moreover HL7 International and CEN/TC 251 will manage the expectations of the IPS standards specification among stakeholders, by |
*stipulating the role of the IPS as a foundation for others to extend | *stipulating the role of the IPS as a foundation for others to extend | ||
*justifying the inclusion of items in the IPS within the limited context of cross-border or cross-jurisdiction unscheduled care. | *justifying the inclusion of items in the IPS within the limited context of cross-border or cross-jurisdiction unscheduled care. | ||
| Line 88: | Line 81: | ||
The more relevant consequences of these principles in the template design are: | The more relevant consequences of these principles in the template design are: | ||
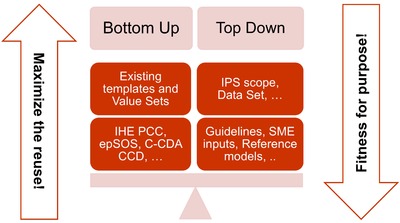
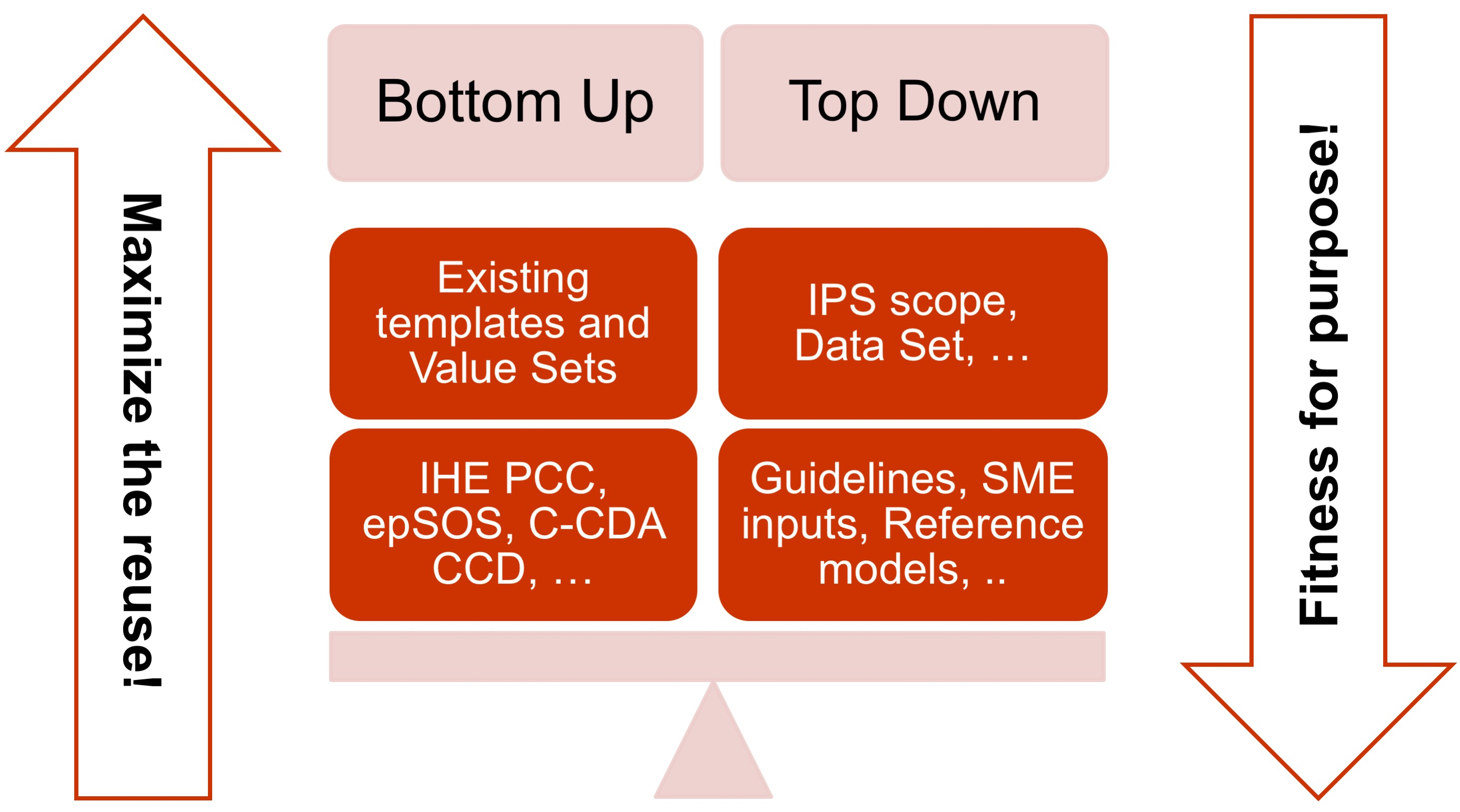
| − | * The adoption of a meet in the middle approach in the | + | * The adoption of a meet in the middle approach in the templates design to balance the need of maximizing the reuse of existing implemented templates (epSOS, C-CDA CCD; IHE PCC…) and facilitate implementation with the need of optimizing the fitness for purpose within the IPS scope. This approach aims to avoid a pure technical exercise of templates harmonization or an academic exercise that does not take in account what is already implemented. |
{{IncludeImage|IPS-meet-in-the-middle.png|400px|90%}} | {{IncludeImage|IPS-meet-in-the-middle.png|400px|90%}} | ||
| − | <ref group="Figure" name="ipsmeetmiddle">The IPS meet-in-the-middle approach</ref>The IPS meet-in-the-middle approach | + | <ref group="Figure" name="ipsmeetmiddle">The IPS meet-in-the-middle approach</ref> The IPS meet-in-the-middle approach |
| − | *Cooperate with the HL7 Terminology Authority and the organizations that own the used code systems (e.g. SNOMED International) to make | + | *Cooperate with the HL7 Terminology Authority and the organizations that own the used code systems (e.g. SNOMED International) to make the IPS value sets available for global use at no cost for implementation of the IPS. |
| − | *When global identifiers are not (or not yet) available, as in the case of the medicinal products, enhance the model proposed for that element with relevant identifying and descriptive attributes that could help the global identification of that element. | + | *When global identifiers are not (or not yet) available, as in the case of the medicinal products, enhance the model proposed for that element with relevant identifying and descriptive attributes that could help with the global identification of that element. |
| − | *Select a set of reference | + | *Select a set of global reference terminologies, with provision for the inclusion of locally used terminologies. |
| − | *Avoid solutions (e.g. identifiers, terminologies, standards) | + | *Avoid solutions (e.g. identifiers, terminologies, standards) that are not yet available for actual global use (even those that are otherwise promising for resolution of well-known issues, such as medicinal product identification). However, the IPS has been already designed, where possible, to be ready to adopt these solutions when they are made available for real use (e.g. the IDMP identifiers) and to already support parts of those solutions that can be used today. |
| − | *Within the scope of the IPS and of the “implementable” principle, attempt to be | + | *Within the scope of the IPS and of the “implementable” principle, attempt to be sufficiently generic in the design of the templates so that the IPS templates are extensible for supporting new scenarios, specific specialties or conditions through template specialization or adaptation mechanisms. |
== Structuring Choices == | == Structuring Choices == | ||
| − | The International Patient Summary is specified as a templated document using HL7 CDA R2. The specification | + | The International Patient Summary is specified as a templated document using HL7 CDA R2. |
| + | The expressiveness of SNOMED CT and other primary terminologies enables this specification to represent the two general categories “condition/activity unknown” and “condition/activity known absent” in a style which is more independent of the underlying syntax (CDA R2 or [http://hl7.org/fhir/STU3/index.html FHIR]), as explained in detail in [[IPS_implementationguide_1#Representing "known absent" and "not known"| section 4.2]]. | ||
| − | To be universally exchangeable, a patient summary must rely on multilingual international reference terminologies. | + | To be universally exchangeable and understood, a patient summary must rely as much as possible on structured data and multilingual international reference terminologies that are licensed at no cost for global use in the International Patient Summary. In the case of SNOMED CT, it is envisioned that SNOMED International could embrace the idea of a globally accessible open and free specification for the International Patient Summary that references a core set of globally accessible and usable value sets licensed at no-cost with the aim to serve the public good. In this spirit, this version of the International Patient Summary defines SNOMED CT as a primary terminology (the meaning of "primary terminology" is explained in [[IPS_implementationguide_1#How_to_use_terminologies_(preferred_binding)| section 4.1]]) and it is used in many of the value sets. To allow, however, a global and free implementation of the IPS this guide does not impose the usage of these SNOMED CT-based value sets. This choice may be revised in future versions. Other primary terminologies used in this specification are LOINC for observations (e.g., laboratory tests) and document sections, UCUM for units of measure, and EDQM Standard Terms for dose forms and routes. Looking at the availability of other globally usable reference terminologies and toward alignment with a future FHIR version of the IPS, in some selected cases FHIR-defined terminologies are recommended. |
| − | This specification adopts ART-DECOR®<ref>ART-DECOR® art-decor.org</ref> as the specification platform for this Implementation Guide and uses the HL7 template exchange format<ref name="teits"/>. This tool and format are increasingly used by several regions, including European countries, and have been adopted by the EU eHealth Digital Service Infrastructure (eHDSI) project for the operational deployment of the EU cross-borders patient summary and ePrescription services. | + | This specification adopts ART-DECOR®<ref>ART-DECOR® art-decor.org</ref> as the specification platform for this Implementation Guide and uses the HL7 template exchange format<ref name="teits"></ref>. This tool and format are increasingly used by several regions, including European countries, and have been adopted by the EU eHealth Digital Service Infrastructure (eHDSI) project for the operational deployment of the EU cross-borders patient summary and ePrescription services. |
| − | Users of the specification can visit the IPS project page in ART-DECOR® to browse the specifications and review examples. | + | Users of the specification can visit the IPS project page in ART-DECOR® to browse the specifications and review examples. Users may also use the tool to validate their IPS instances. |
==Ballot Status of the Document== | ==Ballot Status of the Document== | ||
| Line 122: | Line 116: | ||
*Healthcare providers that populate regional and national patient summaries. | *Healthcare providers that populate regional and national patient summaries. | ||
Technical | Technical | ||
| − | *Vendors of | + | *Vendors of EHR systems for unplanned care management, personal health records and mobile health data applications. |
*System integrators. | *System integrators. | ||
*Organizations that manage regional and national patient summaries. | *Organizations that manage regional and national patient summaries. | ||
| Line 131: | Line 125: | ||
This project relates to other projects and products as: | This project relates to other projects and products as: | ||
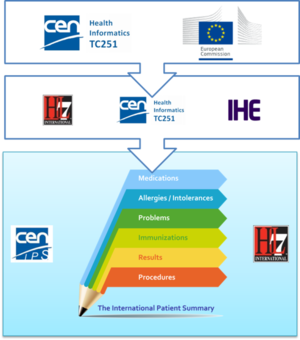
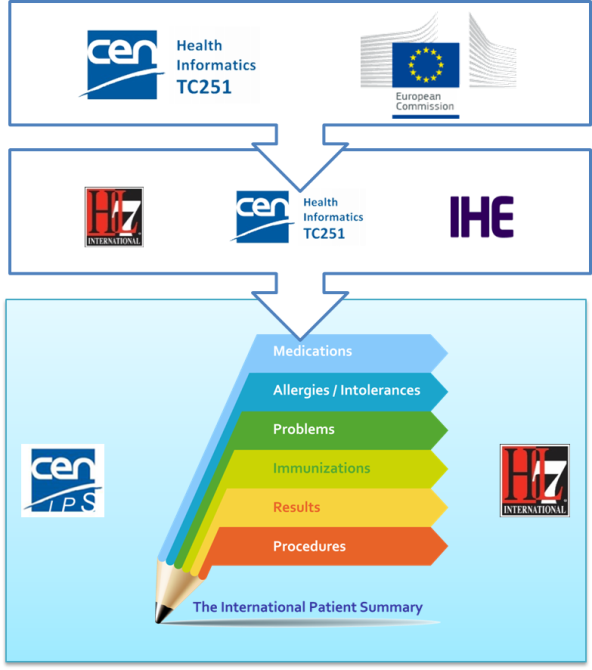
| − | * The '''European Commission CEN/TC 251 Grant Agreement''' “The International Patient Summary Standards Project” (SA/CEN/GROW/EFTA/000/2015-16). <br | + | * The '''European Commission CEN/TC 251 Grant Agreement''' “The International Patient Summary Standards Project” (SA/CEN/GROW/EFTA/000/2015-16). <br> This project has as one of its goal ''“to participate in the creation of an International Patient Summary specification, at a global level, and turn this into a European standard, in line with the Guidelines on Minimum/Nonexhaustive Patient Summary Dataset for Electronic Exchange as adopted by the European eHealth Network"''<br>Under this project two other standard work items have been promoted under CEN/TC 251: |
| − | **The '''CEN/TC 251 “prEN: The Patient Summary for Unscheduled, Cross-border Care”'''. <br | + | **The '''CEN/TC 251 “prEN 17269: The Patient Summary for Unscheduled, Cross-border Care”'''. <br>Its goal is to “formalise the dataset required to share information about the medical background and history of a patient …. It uses the European guidelines (version 2, November 2016) as an official source for the requirements….” <br>Even if it is a European standard it is designed to be applicable in a wider global context. |
| − | **The '''CEN/TC 251 “prTS: The International Patient Summary: Guidance for European Implementation Technical Specification'''. <br | + | **The '''CEN/TC 251 “prTS 17288: The International Patient Summary: Guidance for European Implementation Technical Specification'''. <br>Its goal is to “provide implementation guidance to support the use of the International Patient Summary dataset in a European context” <br>This document is focused on the European cross-country services. |
<div style="padding-left:30px"> | <div style="padding-left:30px"> | ||
{{IncludeImage|CEN IPS Grant.png|300px|60%}} | {{IncludeImage|CEN IPS Grant.png|300px|60%}} | ||
| − | <ref group="Figure" name="ipsmeetinthemiddle">The European Commission CEN/TC 251 Grant Agreement</ref>The European Commission CEN/TC 251 Grant Agreement | + | <ref group="Figure" name="ipsmeetinthemiddle">The European Commission CEN/TC 251 Grant Agreement</ref> The European Commission CEN/TC 251 Grant Agreement |
</div> | </div> | ||
| − | * The '''European eHealth Network Guideline on the electronic exchange of health data under Cross-Border Directive 2011/24/EU. Release 2.''' <ref>EHN Guideline on the electronic exchange of health data under Cross-Border Directive 2011/24/EU. Release 2. https://ec.europa.eu/health/sites/health/files/ehealth/docs/ev_20161121_co10_en.pdf</ref> This Guideline, together with the general guidelines for the electronic exchange of health data under Cross-Border Directive 2011/24/EU, | + | * The '''European eHealth Network Guideline on the electronic exchange of health data under Cross-Border Directive 2011/24/EU. Release 2.''' <ref>EHN Guideline on the electronic exchange of health data under Cross-Border Directive 2011/24/EU. Release 2. https://ec.europa.eu/health/sites/health/files/ehealth/docs/ev_20161121_co10_en.pdf</ref> This Guideline, together with the general guidelines for the electronic exchange of health data under Cross-Border Directive 2011/24/EU, documents the clauses agreed among the European Countries to support the exchange of Patient Summary data for unscheduled care. |
| − | The relationships among these standards are shown in | + | The relationships among these standards are shown in Figure 14 included in the section [[#Conformance clause|Conformance clause]]. |
| − | + | Listed below are other related initiatives: | |
| − | * The '''HL7 Consolidated CDA (C-CDA)''' <ref>http://www.hl7.org/implement/standards/product_brief.cfm?product_id=379 </ref> implementation guide was developed and produced through the joint efforts of HL7, two Sub-Work Groups of the Office of the National Coordinator (ONC) Standards and Interoperability (S&I) Framework — Longitudinal Care Plan (LCP) and Long-Term Post-Acute Care (LTPAC) Transition) — and through the SMART C-CDA Collaborative hosted by ONC and Harvard Medical School. It provides a library of CDA templates for implementing a set of CDA documents. <br | + | * The '''HL7 Consolidated CDA (C-CDA)''' <ref>http://www.hl7.org/implement/standards/product_brief.cfm?product_id=379</ref> implementation guide was developed and produced through the joint efforts of HL7, two Sub-Work Groups of the Office of the National Coordinator (ONC) Standards and Interoperability (S&I) Framework — Longitudinal Care Plan (LCP) and Long-Term Post-Acute Care (LTPAC) Transition) — and through the SMART C-CDA Collaborative hosted by ONC and Harvard Medical School. It provides a library of CDA templates for implementing a set of CDA documents. <br>This is one of the primary sources for this Implementation Guide. |
| − | *The '''IHE Patient Care Coordination''' (PCC) Cross-Enterprise Sharing of Medical Summaries (XDS-MS) <ref>IHE Patient Care Coordination Technical Framework http://ihe.net/Technical_Frameworks/#pcc</ref> – “defines a mechanisms to automate the sharing process between care providers of Medical Summaries, a class of clinical documents that contain the most relevant portions of information about the patient intended for a specific provider or a broad range of potential providers in different settings.” . <br | + | *The '''IHE Patient Care Coordination''' (PCC) Cross-Enterprise Sharing of Medical Summaries (XDS-MS) <ref>IHE Patient Care Coordination Technical Framework http://ihe.net/Technical_Frameworks/#pcc</ref> – “defines a mechanisms to automate the sharing process between care providers of Medical Summaries, a class of clinical documents that contain the most relevant portions of information about the patient intended for a specific provider or a broad range of potential providers in different settings.” . <br>This is one of the primary sources for this Implementation Guide. |
*'''eHealth Digital Service Infrastructure (eHDSI) Patient Summary Service''' <ref>https://ec.europa.eu/cefdigital/wiki/display/EHOPERATIONS/Specifications</ref>.This European initiative operationalizes the work done by the epSOS and EXPAND projects for the implementation of European Cross-border services for the exchange of patient summaries and ePrescriptions. This is one of the primary sources for this Implementation Guide. | *'''eHealth Digital Service Infrastructure (eHDSI) Patient Summary Service''' <ref>https://ec.europa.eu/cefdigital/wiki/display/EHOPERATIONS/Specifications</ref>.This European initiative operationalizes the work done by the epSOS and EXPAND projects for the implementation of European Cross-border services for the exchange of patient summaries and ePrescriptions. This is one of the primary sources for this Implementation Guide. | ||
| − | * The Joint Initiative on SDO Global Health Informatics Standardization '''(JIC) Patient Summary Standards Set''' is a set of health informatics standards and related | + | * The Joint Initiative on SDO Global Health Informatics Standardization '''(JIC) Patient Summary Standards Set''' is a set of health informatics standards and related artifacts that can be used to support the implementation of a Patient Summary <ref>JIC http://www.jointinitiativecouncil.org/index.asp</ref>. The definition of the Patient Summary given by this initiative is a little broader than that adopted by the HL7 and CEN/TC 251 projects, being ““the minimum set of information needed to assure healthcare coordination and the continuity of care” . |
| − | * The '''Data Provenance''' is an ONC S&I | + | * The '''Data Provenance''' is an ONC S&I initiative addressing the “source data” challenge so that trust in the authenticity of the data can help inform decision making. The HL7 CDA® Release 2 Implementation Guide: Data Provenance, Release 1<ref>HL7 CDA® Release 2 Implementation Guide: Data Provenance, Release 1 http://www.hl7.org/implement/standards/product_brief.cfm?product_id=420</ref> is one of the products resulting from the joint efforts of Health Level Seven (HL7) and the Office of the National Coordinator (ONC) Standards and Interoperability Standards and Interoperability Framework-Data Provenance Initiative. |
| − | + | ==Reading Publication Artifacts== | |
| − | + | A reading guide is available that explains the formalism used to express the publication artifacts, i.e. template meta data and template design. For convenience the guide is included in the appendix. (see section [[ #How_to_read_the_table_view_for_templates| 12 How to read the table view for templates]]) | |
| − | ==Reading Publication | ||
| − | A reading guide is available that explains the formalism used to express the publication | ||
Latest revision as of 09:10, 2 July 2018
Contents
Introduction
An International Patient Summary (IPS) document is an electronic health record extract containing essential healthcare information intended for use in the unscheduled, cross-border care scenario, comprising at least the required elements of the IPS dataset. The IPS dataset is a minimal and non-exhaustive patient summary dataset, specialty-agnostic, condition-independent, but readily usable by clinicians for the cross-border unscheduled care of a patient.
Purpose
The goal of this Implementation Guide is to identify the required clinical data, vocabulary and value sets for an international patient summary. The international patient summary is specified as a templated document using HL7 CDA R2. The primary use case is to provide support for cross-border or cross-juridictional emergency and unplanned care.
This specification aims to support:
- Cross-jurisdictional patient summaries (through adaptation/extension for multi-language and realm scenarios, including translation).
- Emergency and unplanned care in any country, regardless of language.
- Value sets based on international vocabularies that are usable and understandable in any country.
- Data and metadata for document-level provenance.
Project Background
This Implementation Guide has drawn upon the results of multiple previous projects on patient summaries (including but not limited to epSOS [1], ONC S&I, Trillium Bridge[2], Sequoia eHealth Exchange [3]), rules and recommendations for vocabularies and value sets (in multilingual settings) and templates for the implementation of international patient summary documents.
The idea of the International Patient Summary has been one of the main results of the 2010 EU/US Memorandum of Understanding through its two operational arms: the European project Trillium Bridge and the Interoperability of EHR work group formed under the ONC Standards and Interoperability Framework (ONC S&I) EU/US eHealth Cooperation Initiative[4]. These initiatives identified the need for common templates and vocabularies for the patient summary.
The Joint Initiative Council (JIC) on SDO Global Health Informatics Standardization has initiated the standard sets project with patient summary as its pilot [5]; and the IPS became one of the main subjects of the new EU / US roadmap , having as a declared goal “to enable a standardized international patient summary (IPS) to be in use by 2020”[6].
The first standardization activity concerning the IPS was initially promoted in April 2014 by ONC within HL7 International. The project was called “INTernational PAtient Summary (INTERPAS)”. In May 2016, the European Commission granted an Agreement with CEN/ TC 251, recognizing the need to effectively support the leadership and active participation in IPS standardization activities. Thanks to the new boost from the European Commission (EC) and ONC a revision of the HL7 project was started in May 2016, as well as the standardization activities in CEN/TC 251 for the European standards on Patient Summaries. Since the beginning of this new phase, the initiatives were envisaged as a single common IPS project supported by different organizations; where the CEN/TC 251 and the HL7 teams worked together, taking in account the inputs of the JIC Standard Sets initiative on Patient Summary, with the common intent of developing coherent set of standards to support the International Patient Summary concept.
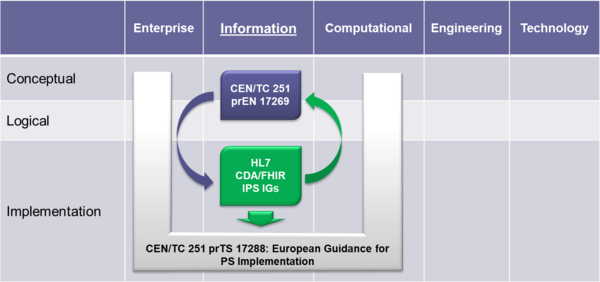
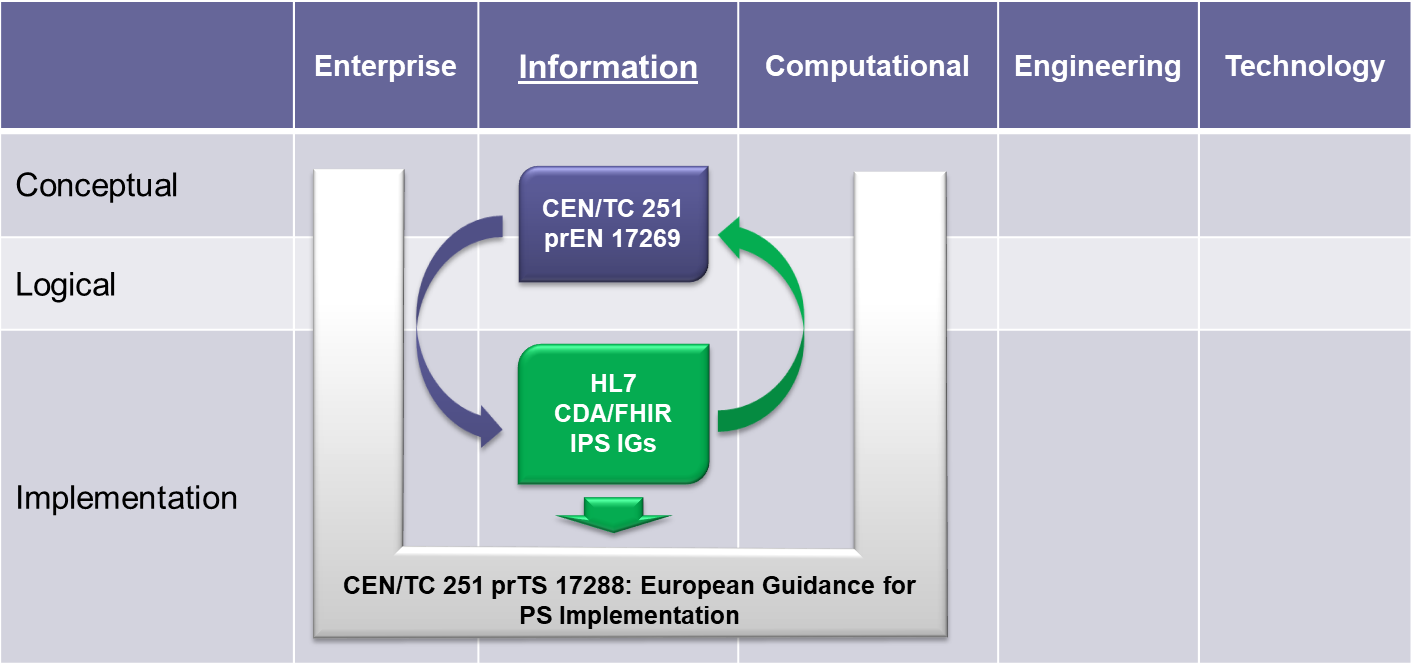
To expedite progress it was also agreed to set up an informal collaboration, promoting a continuous alignment process between the two SDO-specific projects, thanks also to a cross-participation in the project teams. Overlaps have thus been minimized: the CEN/TC 251 activities have been focused on the IPS dataset, formalized by the CEN/TC 251 Draft European standard (prEN) 17269:2018: The Patient Summary for Unscheduled, Cross-border Care" (the CEN/TC 251 prEN 17269:2018 PS in [Figure 1]); the HL7 ones initially on its implementation based on HL7 CDA R2 - this guide - and next on FHIR (the HL7 IPS IGs in [Figure 1]). The figure shows how the products of these standardization activities are placed in the HL7 SAIF Interoperability Matrix.
[Figure 1] IPS Standards in the HL7 SAIF Interoperability Matrix
A formal agreement between HL7 International and CEN/TC 251 has been finally signed in April 2017 in which these organizations established “in order to further the care for citizens across the globe <…> to collaborate on a single, common International Patient Summary (IPS) specification”; and that “the IPS specification shall focus on a minimal and non-exhaustive Patient Summary dataset, which is specialty-agnostic and condition-independent, but still clinically relevant.”.
Scope
As expressed in the introduction, the International Patient Summary is
- a minimal and non-exhaustive patient summary,
- specialty-agnostic,
- condition-independent,
- but readily usable by clinicians for cross-border unscheduled care of a patient.
In this context, minimal and non-exhaustive means that an IPS is not intended to reproduce (the entire) content of an Electronic Health Record (EHR).
Specialty-agnostic means that an IPS is not filtered for a specialty. As an example, allergies are not filtered to the specialty of internal medicine, thus may also include food allergies, if considered to be relevant for, e.g. unplanned care.
Condition-independent means that an IPS is not specific to particular conditions, and focuses on the patient current condition(s).
Furthermore the scope of the IPS is global. Although this is a major challenge, this implementation guide takes various experiences and newer developments into account to address global feasibility as far as possible.
General Principles for this Specification
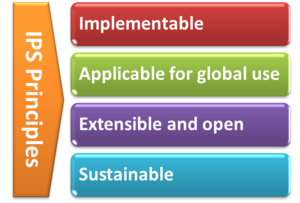
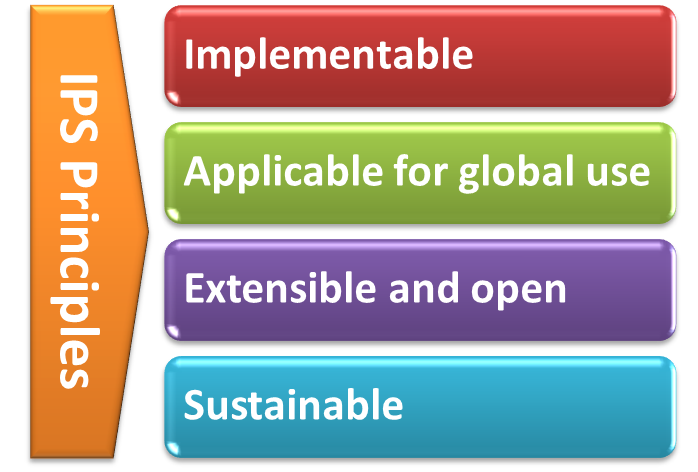
With the formal agreement signed on April 2017, HL7 International and CEN/TC 251 expressed their intent to collaborate under the following principles for the IPS:
[Figure 2] The IPS Principles
- The standards specification for the IPS will be implementable
- Promote (the evolution and convergence of) existing standards
- Rely on solutions that are already implemented or ready for implementation
- Consider new or additional solutions as they become available
- The standards specification for the IPS will be applicable for global use
- Strive for global accessibility of standards for use at no cost
- Strive for a core set of globally accessible and usable terminologies and value sets
- Include free text in addition to the structured codes as needed
- Do not include local solutions in the core specification that are not available in other jurisdictions
- The standards specification will be extensible and open to future use cases and solutions
- The IPS provides common content that can be extended and specialized for other use cases, or localized for specific jurisdictional needs
- The IPS is open to emerging solutions for unresolved issues or improvements
- The standards specification and their implementation must be sustainable through:
- A robust maintenance and update process for the IPS
- A process to ensure clinical validity of the IPS, meeting:
- clinical requirements (including workflow)
- clinical documentation requirements
- information quality requirements
Moreover HL7 International and CEN/TC 251 will manage the expectations of the IPS standards specification among stakeholders, by
- stipulating the role of the IPS as a foundation for others to extend
- justifying the inclusion of items in the IPS within the limited context of cross-border or cross-jurisdiction unscheduled care.
The more relevant consequences of these principles in the template design are:
- The adoption of a meet in the middle approach in the templates design to balance the need of maximizing the reuse of existing implemented templates (epSOS, C-CDA CCD; IHE PCC…) and facilitate implementation with the need of optimizing the fitness for purpose within the IPS scope. This approach aims to avoid a pure technical exercise of templates harmonization or an academic exercise that does not take in account what is already implemented.
[Figure 3] The IPS meet-in-the-middle approach
- Cooperate with the HL7 Terminology Authority and the organizations that own the used code systems (e.g. SNOMED International) to make the IPS value sets available for global use at no cost for implementation of the IPS.
- When global identifiers are not (or not yet) available, as in the case of the medicinal products, enhance the model proposed for that element with relevant identifying and descriptive attributes that could help with the global identification of that element.
- Select a set of global reference terminologies, with provision for the inclusion of locally used terminologies.
- Avoid solutions (e.g. identifiers, terminologies, standards) that are not yet available for actual global use (even those that are otherwise promising for resolution of well-known issues, such as medicinal product identification). However, the IPS has been already designed, where possible, to be ready to adopt these solutions when they are made available for real use (e.g. the IDMP identifiers) and to already support parts of those solutions that can be used today.
- Within the scope of the IPS and of the “implementable” principle, attempt to be sufficiently generic in the design of the templates so that the IPS templates are extensible for supporting new scenarios, specific specialties or conditions through template specialization or adaptation mechanisms.
Structuring Choices
The International Patient Summary is specified as a templated document using HL7 CDA R2. The expressiveness of SNOMED CT and other primary terminologies enables this specification to represent the two general categories “condition/activity unknown” and “condition/activity known absent” in a style which is more independent of the underlying syntax (CDA R2 or FHIR), as explained in detail in section 4.2.
To be universally exchangeable and understood, a patient summary must rely as much as possible on structured data and multilingual international reference terminologies that are licensed at no cost for global use in the International Patient Summary. In the case of SNOMED CT, it is envisioned that SNOMED International could embrace the idea of a globally accessible open and free specification for the International Patient Summary that references a core set of globally accessible and usable value sets licensed at no-cost with the aim to serve the public good. In this spirit, this version of the International Patient Summary defines SNOMED CT as a primary terminology (the meaning of "primary terminology" is explained in section 4.1) and it is used in many of the value sets. To allow, however, a global and free implementation of the IPS this guide does not impose the usage of these SNOMED CT-based value sets. This choice may be revised in future versions. Other primary terminologies used in this specification are LOINC for observations (e.g., laboratory tests) and document sections, UCUM for units of measure, and EDQM Standard Terms for dose forms and routes. Looking at the availability of other globally usable reference terminologies and toward alignment with a future FHIR version of the IPS, in some selected cases FHIR-defined terminologies are recommended.
This specification adopts ART-DECOR®[7] as the specification platform for this Implementation Guide and uses the HL7 template exchange format[8]. This tool and format are increasingly used by several regions, including European countries, and have been adopted by the EU eHealth Digital Service Infrastructure (eHDSI) project for the operational deployment of the EU cross-borders patient summary and ePrescription services. Users of the specification can visit the IPS project page in ART-DECOR® to browse the specifications and review examples. Users may also use the tool to validate their IPS instances.
Ballot Status of the Document
This Implementation Guide is STU with the intention to go normative.
Audience
The audience for this Implementation Guide includes:
Public
- Citizens who want to carry or access their healthcare data for emergency or unplanned care purposes.
Regulatory
- Policy makers such as healthcare payers or government agencies.
- Healthcare information governance authorities and regulatory bodies.
Clinical
- Healthcare providers that offer unscheduled and emergency care.
- Healthcare providers that populate regional and national patient summaries.
Technical
- Vendors of EHR systems for unplanned care management, personal health records and mobile health data applications.
- System integrators.
- Organizations that manage regional and national patient summaries.
Relationships with other projects and guidelines
This guide is one of the products of the International Patient Summary project (see the Project Background section for details). This project relates to other projects and products as:
- The European Commission CEN/TC 251 Grant Agreement “The International Patient Summary Standards Project” (SA/CEN/GROW/EFTA/000/2015-16).
This project has as one of its goal “to participate in the creation of an International Patient Summary specification, at a global level, and turn this into a European standard, in line with the Guidelines on Minimum/Nonexhaustive Patient Summary Dataset for Electronic Exchange as adopted by the European eHealth Network"
Under this project two other standard work items have been promoted under CEN/TC 251:- The CEN/TC 251 “prEN 17269: The Patient Summary for Unscheduled, Cross-border Care”.
Its goal is to “formalise the dataset required to share information about the medical background and history of a patient …. It uses the European guidelines (version 2, November 2016) as an official source for the requirements….”
Even if it is a European standard it is designed to be applicable in a wider global context. - The CEN/TC 251 “prTS 17288: The International Patient Summary: Guidance for European Implementation Technical Specification.
Its goal is to “provide implementation guidance to support the use of the International Patient Summary dataset in a European context”
This document is focused on the European cross-country services.
- The CEN/TC 251 “prEN 17269: The Patient Summary for Unscheduled, Cross-border Care”.
- The European eHealth Network Guideline on the electronic exchange of health data under Cross-Border Directive 2011/24/EU. Release 2. [9] This Guideline, together with the general guidelines for the electronic exchange of health data under Cross-Border Directive 2011/24/EU, documents the clauses agreed among the European Countries to support the exchange of Patient Summary data for unscheduled care.
The relationships among these standards are shown in Figure 14 included in the section Conformance clause.
Listed below are other related initiatives:
- The HL7 Consolidated CDA (C-CDA) [10] implementation guide was developed and produced through the joint efforts of HL7, two Sub-Work Groups of the Office of the National Coordinator (ONC) Standards and Interoperability (S&I) Framework — Longitudinal Care Plan (LCP) and Long-Term Post-Acute Care (LTPAC) Transition) — and through the SMART C-CDA Collaborative hosted by ONC and Harvard Medical School. It provides a library of CDA templates for implementing a set of CDA documents.
This is one of the primary sources for this Implementation Guide. - The IHE Patient Care Coordination (PCC) Cross-Enterprise Sharing of Medical Summaries (XDS-MS) [11] – “defines a mechanisms to automate the sharing process between care providers of Medical Summaries, a class of clinical documents that contain the most relevant portions of information about the patient intended for a specific provider or a broad range of potential providers in different settings.” .
This is one of the primary sources for this Implementation Guide. - eHealth Digital Service Infrastructure (eHDSI) Patient Summary Service [12].This European initiative operationalizes the work done by the epSOS and EXPAND projects for the implementation of European Cross-border services for the exchange of patient summaries and ePrescriptions. This is one of the primary sources for this Implementation Guide.
- The Joint Initiative on SDO Global Health Informatics Standardization (JIC) Patient Summary Standards Set is a set of health informatics standards and related artifacts that can be used to support the implementation of a Patient Summary [13]. The definition of the Patient Summary given by this initiative is a little broader than that adopted by the HL7 and CEN/TC 251 projects, being ““the minimum set of information needed to assure healthcare coordination and the continuity of care” .
- The Data Provenance is an ONC S&I initiative addressing the “source data” challenge so that trust in the authenticity of the data can help inform decision making. The HL7 CDA® Release 2 Implementation Guide: Data Provenance, Release 1[14] is one of the products resulting from the joint efforts of Health Level Seven (HL7) and the Office of the National Coordinator (ONC) Standards and Interoperability Standards and Interoperability Framework-Data Provenance Initiative.
Reading Publication Artifacts
A reading guide is available that explains the formalism used to express the publication artifacts, i.e. template meta data and template design. For convenience the guide is included in the appendix. (see section 12 How to read the table view for templates)
- ↑ The epSOS Project http://epsos.eu/
- ↑ The Trillium Bridge Project http://www.trilliumbridge.eu
- ↑ The Sequoia Project https://sequoiaproject.org/
- ↑ Memorandum of Understanding between the United States Department of Health and Human Services and the European Commission on Cooperation Surrounding Health Related Information and Communication Technologies http://ec.europa.eu/newsroom/dae/document.cfm?doc_id=1784
- ↑ http://www.jointinitiativecouncil.org/news/JIC_Standards_Set_development_20160101_v1.00.pdf
- ↑ Transatlantic eHealth/health IT Cooperation Roadmap http://ec.europa.eu/newsroom/dae/document.cfm?doc_id=12123
- ↑ ART-DECOR® art-decor.org
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedteits - ↑ EHN Guideline on the electronic exchange of health data under Cross-Border Directive 2011/24/EU. Release 2. https://ec.europa.eu/health/sites/health/files/ehealth/docs/ev_20161121_co10_en.pdf
- ↑ http://www.hl7.org/implement/standards/product_brief.cfm?product_id=379
- ↑ IHE Patient Care Coordination Technical Framework http://ihe.net/Technical_Frameworks/#pcc
- ↑ https://ec.europa.eu/cefdigital/wiki/display/EHOPERATIONS/Specifications
- ↑ JIC http://www.jointinitiativecouncil.org/index.asp
- ↑ HL7 CDA® Release 2 Implementation Guide: Data Provenance, Release 1 http://www.hl7.org/implement/standards/product_brief.cfm?product_id=420
Cite error: <ref> tags exist for a group named "Figure", but no corresponding <references group="Figure"/> tag was found, or a closing </ref> is missing