Difference between revisions of "MD2"
| Line 27: | Line 27: | ||
</p> | </p> | ||
<p> | <p> | ||
| − | + | </html>[[File:ab46ff5a-a39f-4ab4-86ff-5aa39f6ab401.png]]<html> | |
</p> | </p> | ||
<p> | <p> | ||
| Line 41: | Line 41: | ||
</p> | </p> | ||
<p> | <p> | ||
| − | + | </html>[[File:6534e58b-8c24-4aab-b4e5-8b8c244aabcb.png]]<html> | |
</p> | </p> | ||
<p> | <p> | ||
Revision as of 14:23, 6 August 2024
The use of medication statements in the summary
A medication list may strongly vary depending on the context of use (e.g. support for prescription or dispensation, drug reconciliation, etc. ) and on the type of information reported (e.g. patient-reported medication, prescribed, dispensed or administered medications, active or past medications, etc.).
This is still true also for the medication summary in a Patient Summary. It could be, for instance, a list of "Relevant prescribed medicines whose period of time indicated for the treatment has not yet expired whether it has been dispensed or not, or medicines that influence current health status or are relevant to a clinical decision " (European eHN Guideline on the electronic exchange of health data under Cross-Border Directive 2011/24/EU: Patient Summary. Release 3.3.; a list of actually dispensed medications; a list of relevant medications for the patient (IHE PCC, IHE Patient Care Coordination Technical Framework http://ihe.net/Technical_Frameworks/#pcc); or conversely, it could be a complete history including the full patient's prescription and dispensation history and information about intended drug monitoring (C-CDA [HL7 C-CDA Implementation Guide DSTU R2.1 http://www.hl7.org/implement/standards/product_brief.cfm?product_id=379]).
Moreover, for the scope of the International Patient Summary, it is important to know what medications are being taken by or have been given to a patient; without necessarily providing all the details about the medication order, supply, administration or monitoring. This information need, in line with the IPS principle of minimum non exhaustive data, is well expressed by the concept of Medication Statement (see https://www.hl7.org/fhir/medicationstatement.html). The IPS medication summary is therefore typically a list of relevant medication statements, possibly built from either a prescription list or a dispensation list. In fact, in many practical cases data included in a Patient Summary are derived from the list of the medicines prescribed by a GP and recorded in its EHR-S; or extracted from a regional/national prescribing and/or dispensation systems. In these cases, data obtained from actual dispensation and/or prescription records can be still recorded in the IPS as statements and the original request, supply or administration records referred by the statement if really needed.
Medicinal Product Identification
The identification of medicinal products is quite easily solved within a single jurisdiction relying on local drug databases. In contrast, it is one of the major open issues for eHealth services across jurisdictions.
The set of ISO standards called IDMP [IDMP standards https://www.idmp1.com/idmp-standards] – designed initially for the regulatory scope, but hopefully extensible to other domains – are the most promising solution for solving this known issue, as also highlighted by the European project UNICOM. The completion of the IDMP implementation guides, the deployment of the needed supporting services, and the development of some companion standards that will allow the seamless flow of the IDMP identifiers and attributes from the regulatory space to the clinical world (and back) are however still in progress. Additional time is needed before these identifiers and attributes will be available in full for practical use.
Following therefore the IPS principles of “implementability”, “openness" and "extensibility”, the solution proposed here does not rely on IDMP identifiers. Nonetheless, it takes into account, however, relevant IDMP identifiers and attributes which are already usable in the IPS, namely the Pharmaceutical Product Identifiers (PhPIDs), the Medicinal Product Identifier (MPID), and the Medicinal Product Package Identifier (PCID).
Note: IDMP Medicinal Product (MPID) and Medicinal Product Package (PCID) identifiers depend on the market authorization. The “same” product might therefore have different IDs if different authorizations have been received in different countries, while the PhPID should be the same. For the purpose of the IPS, future standards and implementation guides should define global product identifiers that do not depend on the drug registration process (as the Virtual Medicinal Product in SNOMED CT) and relate to IDMP identifiers.
Thus, in the absence of a global identification system for medicinal products, the solution proposed here is based on the approach initially adopted by the European cross-border services (epSOS and currently by the eHDSI project), reused by the IHE Pharmacy templates and more recently adopted (for specific cases) by the HL7 Pharmacy Medication statement templates. The main idea is to integrate local drug identifiers (e.g. product codes) with all the relevant identifying and descriptive attributes that may help the receiver to understand the type of product the sender is referring to, e.g.: active ingredients, administrative dose forms, strength, route of administration and package description.
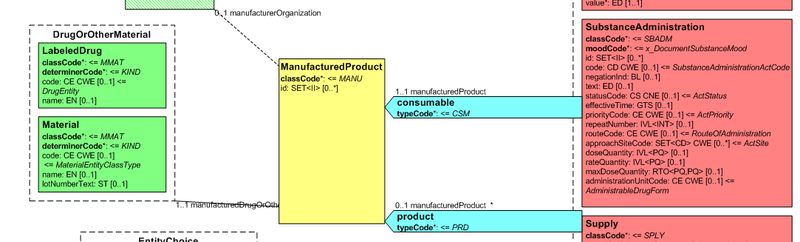
Medication data is usually represented in the CDA Templates using the manufacturedMaterial class, which includes a code and a name to describe any level of the product: packaged product, medicinal product, classes or clusters or products, and so on.
This information is not however sufficient for covering the requirements of the IPS.
Figure: Representation of medicines in CDA
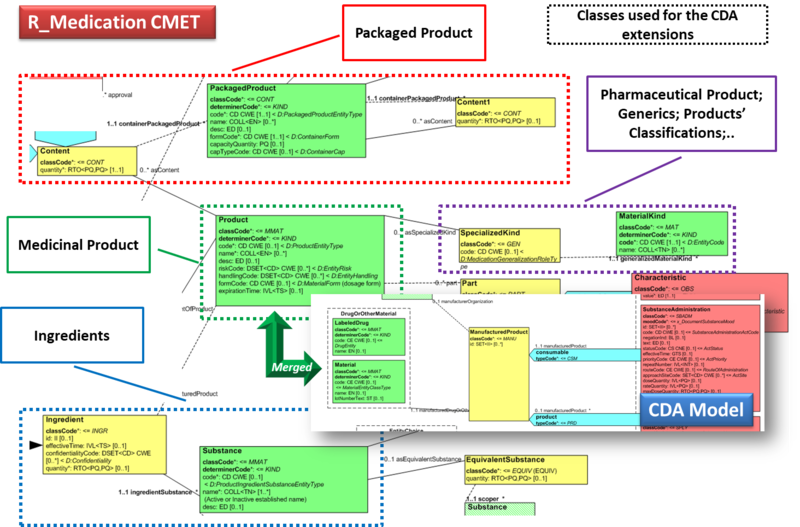
Hence, in order to describe these attributes the CDA model has been extended enhancing the Manufactured Material class with attributes and relationships derived from the latest published version of the R_Medication CMET based on the HL7 Common Product Model (“The common product model is used to improve the alignment between the different representations of products used within the body of HL7 Version 3 models. One goal of this effort is to make it possible to have a single representation, which could then be used as a common message element type (CMET) across those models.”) .
The next figure provides an overview of the CDA model extensions, approach adopted and further developed by the HL7 CDA® R2 Implementation Guide: Pharmacy Templates.
Starting from this IG version, the HL7 CDA IPS medication templates have been defined as specialization of the Pharmacy ones, adopting the pharm (urn:hl7-org:pharm) extensions.
Figure: Extension of the CDA model from the content of the R_Medication CMET.